COVID-19
What is it?
COVID-19 disease is caused by infection with the SARS-CoV-2 virus of which there are many different strains and subvariants. In 2024, the Omicron variant is the predominant strain circulating globally.
Since its emergence in 2019, COVID-19 disease has had vast impacts on society. In addition to the public health implications, the economic, social and mental health effects have been and continue to be significant.
What to look for?
Infections can be asymptomatic or can result in a spectrum of symptoms, from mild respiratory symptoms to more serious disease affecting multiple organs and systems. Severe disease can lead to hospitalisation, mechanical ventilation, and can be fatal.
Common symptoms of acute infection can include rhinorrhoea (runny nose), sneezing, headache, sore throat and fatigue. The symptoms of fever, loss of smell or taste and persisting cough seen in infections with previous variants (Alpha, Beta, Gamma, Delta) are less likely to occur in infections with the Omicron variant.
Long COVID is a chronic condition that can occur following acute infection. It is the persistence of symptoms for greater than 3 months after an initial infection that cannot be attributed to other causes. Long COVID can have a huge impact on an individual’s quality of life, mental health and ability to participate in work or schooling. Long COVID is more common in people who have experienced severe COVID-19 disease, those who had underlying medical conditions prior to infection (e.g. hypertension, chronic lung disease, diabetes, obesity) and those who are unvaccinated.
Paediatric Multisystem Inflammatory Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS), or Multisystem Inflammatory Syndrome in Children associated with COVID-19 (MIS-C), is a newly described condition that has been reported in children in the first 2-6 weeks after COVID-19 infection. It is an inflammatory condition, similar to Kawasaki disease, and is characterised by rash, fever, shock and abdominal pain. Children experiencing PIMS-TS almost always require hospitalisation for treatment.
How is it transmitted?
COVID-19 is transmitted through the inhalation of virus particles made airborne when an infected person coughs, sneezes, breathes, speaks or sings. Droplets containing virus particles can contaminate surfaces and can be spread when a person touches these surfaces and then touches their nose, mouth or eyes. Poorly ventilated settings can also contribute to the spread of COVID-19 because aerosol particles can remain suspended in the air for several hours longer than in well-ventilated settings.
The incubation period for the disease is 1 to 14 days, with most individuals displaying symptoms 3 days after being exposed. Individuals are most infectious in the 2 days before their symptoms begin and the early stages of their illness. People with asymptomatic disease can still infect others.
Epidemiology
Since 2019, over 774 million infections have been reported worldwide contributing to more than 7 million deaths. True figures are likely to be much higher. Serosurveys indicate that by December 2022 more than two thirds of adult Australians had been infected with SARS-CoV-2.
Individuals with immunocompromise, advancing age (particularly > 70 years), obesity, respiratory conditions, heart disease, diabetes, renal disease, liver disease, neurological conditions and disability are more likely to experience severe symptoms if infected with SARS-CoV-2. Pregnancy in unvaccinated people is also recognised as a risk factor for developing severe disease; however, this risk has declined substantially since the Omicron variant became the predominant circulating strain.
Certain occupations, such as working in healthcare, increase likelihood of exposure to SARS-CoV-2 and therefore infection.
Prevention
Strategies to reduce risk of transmission of COVID-19 disease include standard precautions such as hand hygiene, wearing masks when remaining socially distant is not possible and ensuring indoor spaces have good ventilation.
While natural infection does provide some immunity, it is not lifelong, and the emergence of newer strains contributes to repeat infections.
Vaccination aims to reduce the severity of COVID-19 symptoms and the need for hospitalisation. There are 2 vaccine brands available for use within Australia:
- Comirnaty (Pfizer) vaccine containing nucleoside-modified mRNA encoding the spike glycoprotein of SARS-CoV-2
- Spikevax (Moderna) vaccine containing nucleoside-modified mRNA encoding the spike glycoprotein of SARS-CoV-2
As newer variants of the SARS-CoV-2 virus emerge, vaccines are updated to target the strains that are circulating; where possible, these should be used preferentially.
Table 1: Vaccine brands and type available for use by age group
| Age Group | Vaccine brand and type | ||||||
| Comirnaty (Pfizer) Omicron XBB.1.5* | Comirnaty (Pfizer) Original [MDV-maroon cap]* | Comirnaty (Pfizer) Original [MDV-orange cap] | Comirnaty (Pfizer) Omicron XBB.1.5 [MDV-light blue cap] | Comirnaty (Pfizer) Original/Omicron BA4/5 [MDV-grey cap] | Comirnaty (Pfizer) Omicron XBB.1.5 [MDV-dark grey cap] | Spikevax Omicron XBB.1.5 [pre-filled syringe] | |
| < 6 months | |||||||
| 6 months - 4 years | ✓* | ✓* | |||||
| 5 - 11 years | ✓ | ✓ | |||||
| ≥ 12 years | ✓ | ✓ | ✓ |
shaded boxes indicate that vaccine is not available for use in this age group.
shaded boxes indicate preferred vaccines in this age group.
✓ may be used in this age group.
* Comirnaty (Pfizer) Omicron XBB.1.5 is registered for use in individuals aged 6 months to 4 years and supply is anticipated in early 2024. When stock of this formulation becomes available this vaccine will be preferentially recommended in this age group over the Original [Maroon cap] vaccine.
Primary course
Immunocompetent people aged 5 years and over require a single dose of vaccine to complete their primary course.
People aged 6 months and over with severe immunocompromise, and children aged 6 months to 4 years with increased risk of severe disease, require 2 doses, 8 weeks apart. A 3rd dose may be considered based on individual circumstances.
Further doses
Following receipt of a primary course, recommendations for further doses vary depending on the age of the individual and their risk factors for severe disease.
Table 2: 2024 recommendations for COVID-19 booster doses (adapted from ATAGI statement on the administration of COVID-19 vaccines in 2024)
| Age group | Severe immunocompromise | Immunocompetent |
| < 6 months | ||
| 6 months - 4 years | Not recommended | Not recommended |
| 5 - 17 years | Dose can be considered every 12 months | Not recommended |
| 18 - 64 years | Dose recommended every 12 months, can be considered every 6 months | Dose can be considered every 12 months |
| 65 - 74 years | Dose recommended every 12 months, can be considered every 6 months | Dose recommended every 12 months, can be considered every 6 months |
| ≥ 75 years | Dose recommended every 6 months | Dose recommended every 6 months |
Side effects following vaccination
Common side effects
Most side effects following COVID-19 vaccination are mild and can include pain at the injection site, fatigue, headache, lymphadenopathy and fever.
Rare side effects
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining around the heart) are rare conditions that have been reported following administration of COVID-19 vaccines. They are most commonly associated with viral infections (including COVID-19 disease) but can also be triggered by other factors such as medications and autoimmune conditions. In the setting of vaccination, the peak risk group for myocarditis is young adult males aged between 16 and 24 years following a second dose of vaccination. Pericarditis occurring after vaccination is more common in the 20 to 45 year old age group for both males and females.
Thrombosis with thrombocytopenia (TTS) was a rare condition that was reported to occur in people who had previously received the COVID-19 vaccine Vaxzevria (AstraZeneca). Since March 2023, Vaxzevria is no longer available for use in Australia.
Commonly asked questions
Can pregnant or breastfeeding people receive COVID-19 vaccines?
Yes it is safe to administer COVID-19 vaccines at any stage of pregnancy. Due to an increased risk of severe outcomes for pregnant women and their unborn babies it is recommended that any unvaccinated pregnant people receive a primary course of COVID-19 vaccination during pregnancy. Administration of further doses can also be considered if they are due.
Those who are breastfeeding can receive COVID-19 vaccines, and do not need to stop breastfeeding before or after being vaccinated.
Real world surveillance of international data on mRNA COVID-19 vaccine administration in pregnant people has shown no significant safety concerns for either the mother or the baby. Further to this, antibodies have been detected in the cord blood and breastmilk of vaccinated people, suggesting a transfer of protection to the baby.
Where can I access COVID-19 vaccines?
Individuals aged ≥ 5 years can access COVID-19 vaccines through GP clinics and some pharmacies. Children aged 6 months to 4 years can receive COVID-19 vaccines through hospital immunisation services at Monash Children’s Hospital, the Royal Children’s Hospital and Joan Kirner Women’s and Children’s Hospital (Sunshine). To find your closest provider and make an appointment visit the Health direct website.
What is the recommended interval between COVID-19 infection and vaccination?
An interval of 6 months between COVID-19 infection and any COVID-19 vaccination is recommended.
Can COVID-19 vaccines be administered on the same day as other vaccines?
COVID-19 vaccines can be co-administered (given on the same day) with other vaccines, including influenza vaccines for individuals aged 5 years and over. An interval of 7 to 14 days between vaccines is preferred for children aged 6 months to 4 years to avoid the possibility of adverse events such as fever. However, if this is logistically challenging, coadministration can occur.
Due to both vaccines carrying a small risk of developing myocarditis, in circumstances where ACAM 2000 and COVID 19 vaccination is required, an interval of 4 weeks could be considered.
Resources
- MVEC: Vaccine platforms
- MVEC: Immunosuppression and vaccines
- Victorian COVID-19 vaccine safety report
- ATAGI clinical guidance on COVID-19 vaccine administration errors
- Australian Government Department of Health and Aged Care: International COVID-19 vaccines recognised by Australia
- Australian Immunisation Handbook: COVID-19
- CDC: Long COVID or post COVID conditions
Authors: Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: March 2024
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Cancer immunisation guideline: vaccine recommendations following chemotherapy and haematopoietic stem cell transplant
Background
Individuals undergoing cancer treatments (eg. chemotherapy, immunosuppressive therapies or haematopoietic stem cell transplants (HSCT)) are at a higher risk of contracting infectious diseases due to their malignancy, as well as the immune suppression caused by their treatment. Immune suppression can result in an inability to fight new infections, as well as result in the loss of previous immune memory from past infections and vaccines.
Generally speaking, vaccines are withheld during cancer therapy due to an inability to create an effective immune response. The exception to this would be influenza vaccines and COVID-19 vaccines. Individuals with severe neutropenia should not receive vaccines due to the risk of febrile neutropenia.
Following the completion of treatment, individuals are recommended to either be re-vaccinated completely or receive booster doses of vaccines to ensure effective protection against vaccine preventable diseases. The recommendations for vaccine doses/schedules may vary according to type of cancer treatment received, the age of the recipient and any other co-morbidities.
The recommendations for the vaccination of children ≤ 18 years following cancer treatment (within 2 years of completing treatment) are outlined below.
Timing
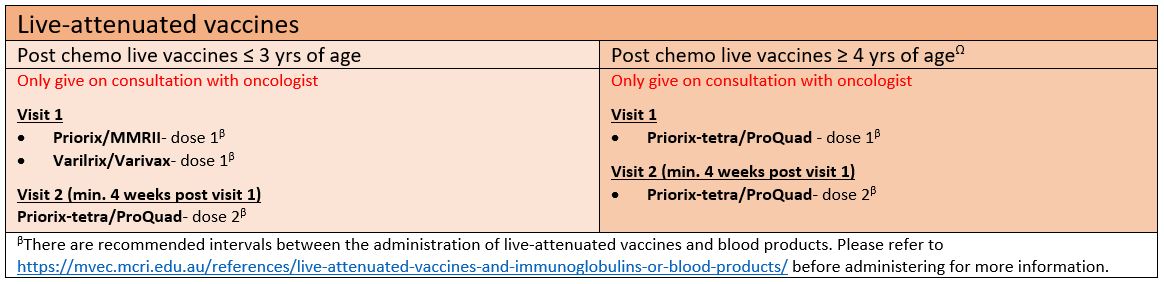
Inactivated vaccines following cancer treatment can be administered from 6 months after the completion of treatment and if the underlying illness is in remission. Live-attenuated vaccines should only be given in consultation with the patient’s treating oncologist.
Post-chemotherapy immunisation guideline
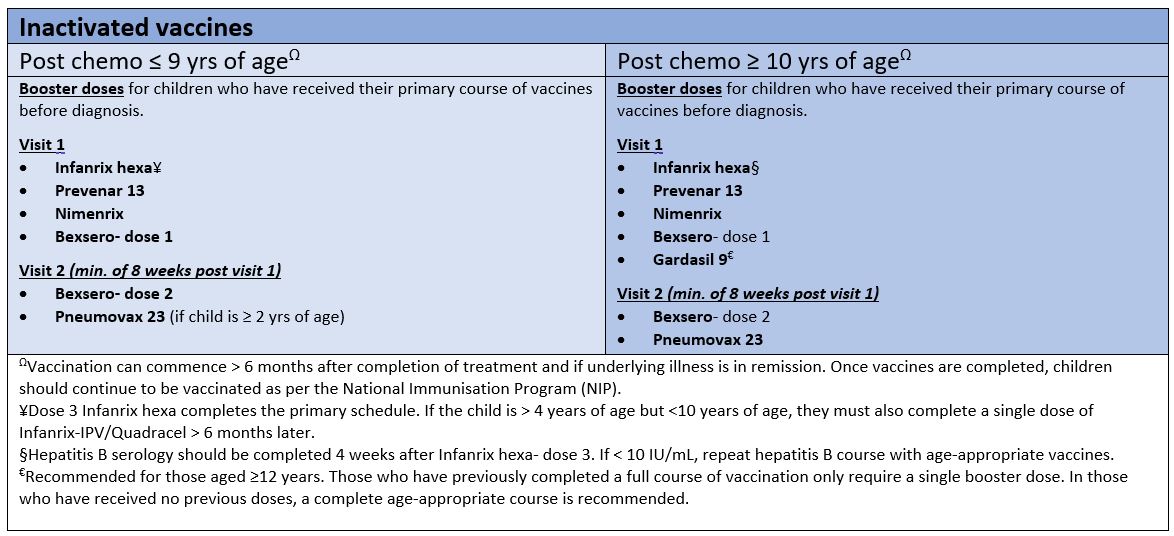
Inactivated vaccines
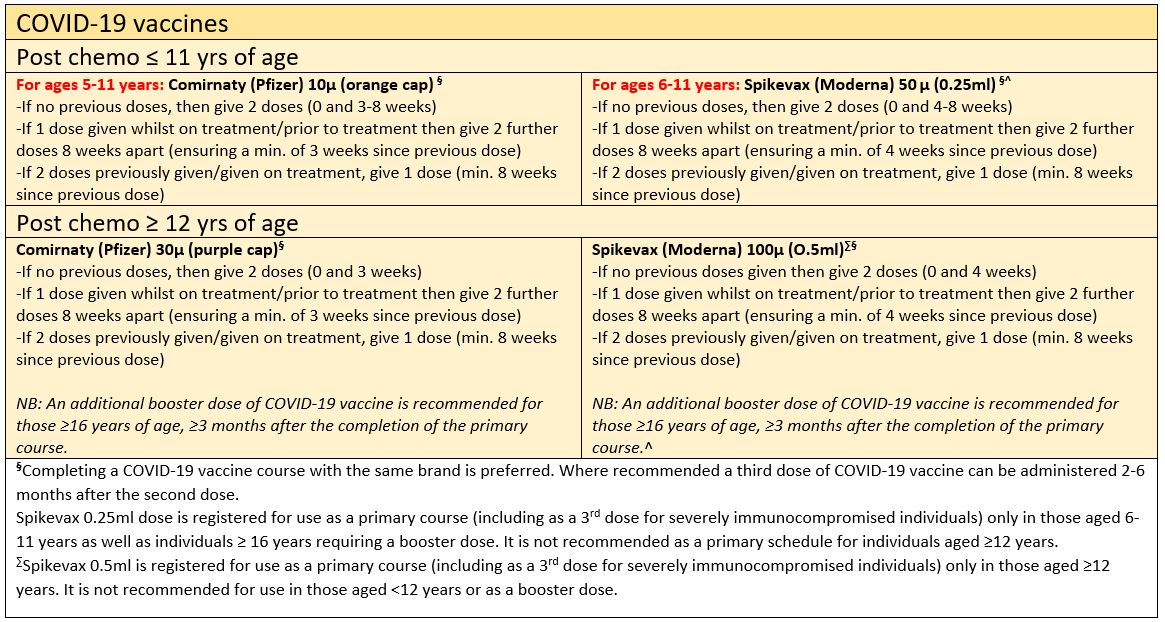
COVID-19 vaccines
Live-attenuated vaccines
Post-HSCT immunisation guideline
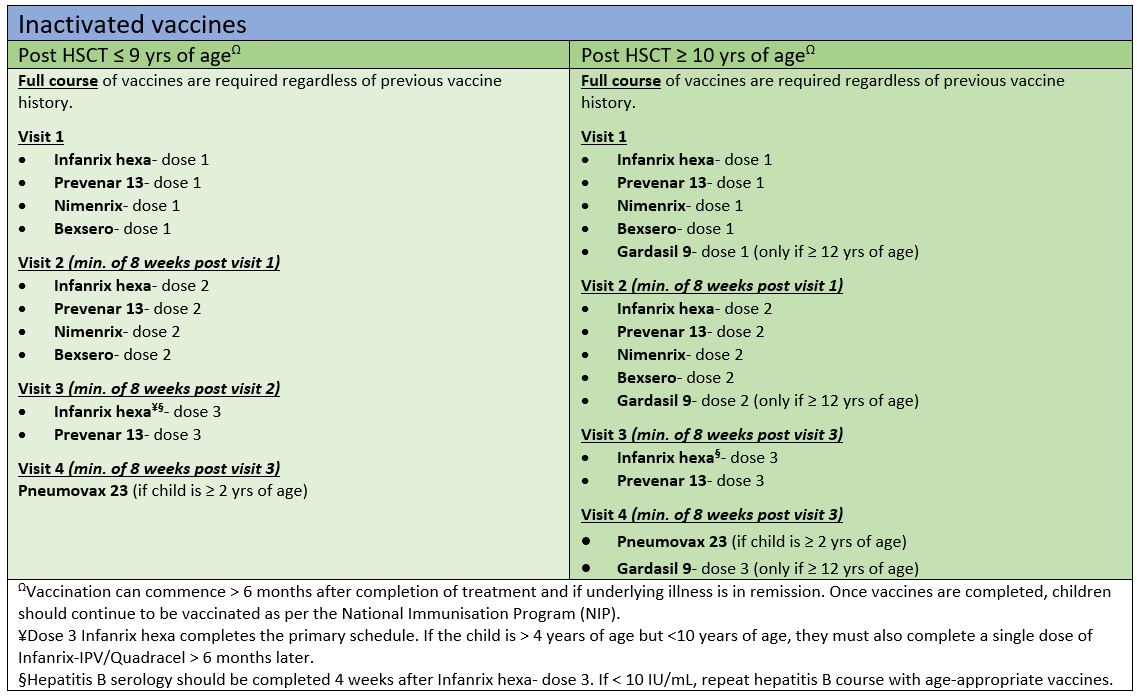
Inactivated vaccines
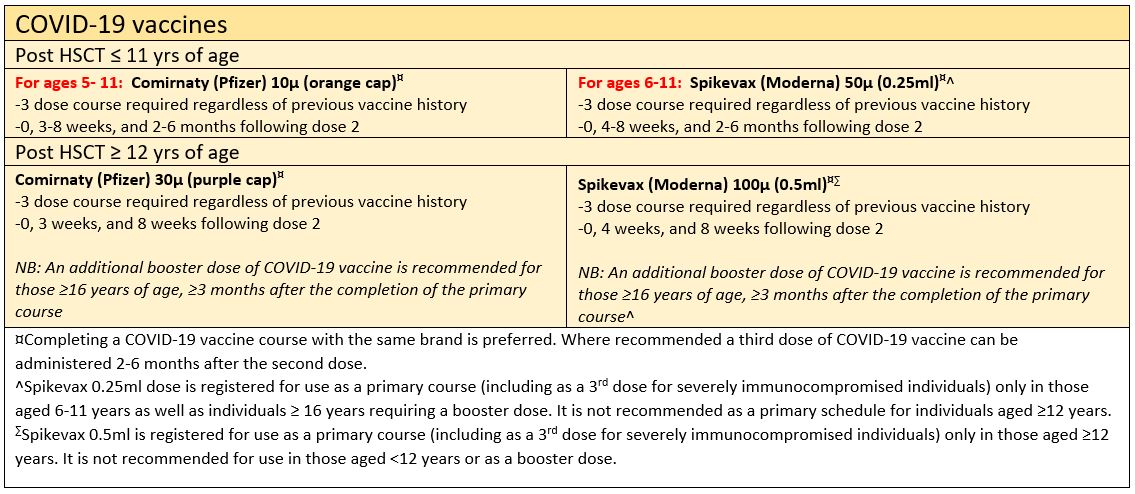
COVID-19 vaccines
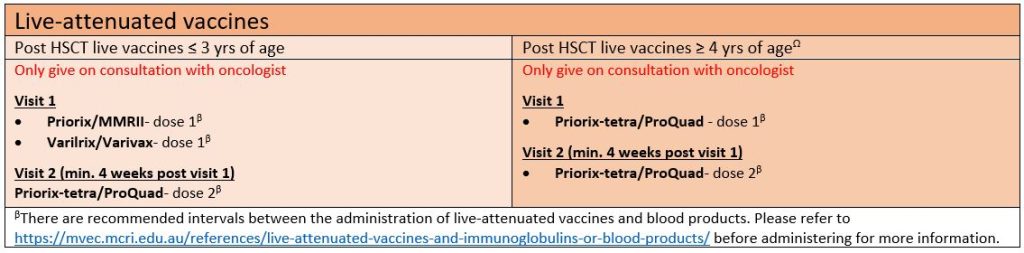
Live-attenuated vaccines
Resources
- MVEC: Post-HSCT immunisation guideline March 2022 (PDF version)
- MVEC: Post chemotherapy immunisation guideline March 2022 (PDF version)
- MVEC: Influenza vaccine recommendations
- MVEC: COVID-19 vaccination in children and adolescents
- MVEC: Needle phobia
- MVEC: Live-attenuated vaccines and immunoglobulins or blood products
Authors: Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Teresa Lazzaro (Consultant Paediatrician, Royal Children’s Hospital)
Date: March 10, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
COVID-19 vaccine compensation scheme
As Australia aims to roll out a COVID-19 vaccine to everyone age eligible, it means that a number of individuals may experience a serious adverse event following immunisation (AEFI). Supporting these vaccinees is important and providing compensation to individuals who have experienced a vaccine injury is a strategy that has been used widely, for a long period of time, in countries such as the United States, UK and New Zealand to promote vaccine confidence and maintain vaccination rates. It is not a concept designed to suggest causation or fault, but a scheme to promote trust in vaccination programs as well as provide vaccine manufacturers with the reassurance to produce vaccines without fear of legal action.
An example of a serious AEFI requiring hospitalisation is thrombosis with thrombocytopenia syndrome (TTS) following Vaxzevria (AstraZeneca), with the symptoms experienced resulting in a significant impact on a vaccine recipient.
The No Fault COVID-19 Indemnity Scheme is funded by the Commonwealth government and has been introduced to allow Australians who have been significantly impacted by an adverse event following a COVID-19 vaccine to apply for access to financial assistance. There is currently no compensation scheme within Australia for applications relating to non-COVID-19 vaccines.
What is involved?
The No Fault COVID-19 Indemnity Scheme will run for 2 years and cover the cost of injuries ($1,000 and above) in situations where a serious adverse reaction has been caused by a COVID-19 vaccination. A full list of qualifying conditions as well as harm not covered under the scheme can be found at COVID-19 vaccine claims scheme- Overview.
Any vaccine encounter which has occurred since February 2021 is covered under this scheme. Individuals vaccinated within Australia, as well as those overseas as part of the Australian Government Overseas Network (AGON) COVID-19 vaccine rollout managed by DFAT are eligible.
Which vaccines are covered under this scheme?
All TGA approved COVID-19 vaccines are covered under this scheme. Comirnaty (Pfizer), Spikevax (Moderna), Vaxzevria (AstraZeneca) and Nuvaxovid (Novavax) are currently in use within Australia;. COVID-19 Vaccine Janssen is also approved by the TGA however is not in use in Australia.
How to make a claim
Affected individuals (or an individual acting on their behalf) can submit their interest in making a claim via Register your interest in the COVID-19 Vaccine Claims Scheme.
Each claim is assessed by independent experts for accuracy and legitimacy. Evidence required in a claim includes:
- details of the injury (including any medical documentation relating to its likely relationship to a COVID-19 vaccination)
- hospitalisation
- medical costs
- lost wages.
Resources
- Australian Government Department of Health: COVID-19 vaccine claims scheme
- Australian Government Department of Health: Register your interest in the COVID-19 vaccine claims scheme
- COVID-19 Vaccine Claims Scheme- Frequently asked questions
- COVID-19 vaccination: After your Spikevax (Moderna) vaccine
- COVID-19 vaccination: After your Comirnaty (Pfizer) vaccine
- COVID-19 vaccination: After your Vaxzevria (AstraZeneca) vaccine
- COVID-19 vaccination: After your Nuvaxovid (Novavax) vaccine
Authors: Rachael McGuire (MVEC Education Nurse Coordinator), Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: February 1, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
COVID-19 vaccines and allergy
Background
Hypersensitivity/allergic reactions following immunisation can be classified as:
- Urticarial – a red, itchy skin rash often referred to as hives, which characteristically has a central raised white wheal surrounded by an area of redness
- Non-urticarial rash – skin changes that don’t involve hives
- Angioedema – swelling in the deeper layers of the skin
- Generalised allergic reaction – involving symptoms like vomiting and diarrhoea
- Anaphylaxis – a sudden onset and rapid progression of symptoms involving the skin, as well as systemic symptoms respiratory and/or cardiovascular systems
Suspected hypersensitivity reactions, particularly non-urticarial skin rashes following immunisation, are common, however true vaccine allergy, where a person is contraindicated from being immunised with the same vaccine in the future, is rare (in most studies reported as less than 1 case per million doses). For further information on diagnosing hypersensitivity reactions and anaphylaxis please refer to Guidance for differentiating anaphylaxis and acute stress response for vaccine providers.
Post-licensure surveillance of COVID-19 vaccines show anaphylaxis following administration of Vaxzevria (AstraZeneca) occurring at similar rates to routine vaccines [refer to TGA: AstraZeneca ChAdOx1-S COVID-19 vaccine]. Anaphylaxis following Comirnaty (Pfizer), whilst still extremely rare, occurs at a slightly higher rate of approximately 4.7 cases per million doses. Data from the US has shown that Spikevax (Moderna) has a rate of anaphylaxis with approximately 2.5 cases per million. Most of these cases (89%) occurred within 30 minutes of vaccination and 26% had a history of prior anaphylaxis.
A confirmed vaccine allergy usually requires a specialist consultation with a vaccine allergy specialist, often with specific testing or a vaccine challenge under supervision.
All COVID-19 immunisation hypersensitivity/allergic reactions should be reported to your state’s vaccine safety reporting service. In Victoria this is SAEFVIC. SAEFVIC staff may direct the report to an immunisation specialist or alternatively to a vaccine allergy specialist within the VicSIS network (a network of specialist immunisation clinics in Victoria), as appropriate.
Allergy to components of COVID-19 vaccines
Polyethylene Glycol (PEG)
PEG is an ingredient contained in mRNA COVID-19 vaccines (Comirnaty (Pfizer) and Spikevax (Moderna)). It is also a commonly used ingredient of other medications, hand sanitisers, cosmetics, bathroom products and colonoscopy preparation products, routinely used within Australia. Whilst it is uncertain whether PEG contained in mRNA vaccines may trigger anaphylaxis, additional precautions are required prior to administration.
It is recommended people with a history of confirmed or suspected allergy to PEG seek specialist advice from an immunology/allergy/vaccination specialist regarding the safety of vaccination.
NB: Vaccination with the Comirnaty (Pfizer) and Spikevax (Moderna) is contraindicated in people with documented anaphylaxis to PEG.
Polysorbate 80
Polysorbate 80 is chemically related to Polyethylene Glycol and is an ingredient in both Vaxzevria (AstraZeneca) and Nuvaxovid (Novavax).
If there is a history of confirmed or suspected allergy to Polysorbate 80 it is recommended that specialist advice be sought from an immunology/allergy/vaccination specialist regarding the safety of administering either vaccine.
NB: Vaccination with Vaxzevria (AstraZeneca) or Nuvaxovid (Novavax) is contraindicated in people with documented anaphylaxis to Polysorbate 80.
Latex
All of the COVID-19 vaccines available for use within Australia (Vaxzevria (AstraZeneca), Comirnaty (Pfizer), Spikevax (Moderna) and Nuvaxovid (Novavax)) can both be administered to people with latex allergies following standard precautions, with a 15-minute post-vaccination observation period.
COVID-19 vaccines and allergies
Reaction following a previous dose of a COVID-19 vaccine
The only two absolute contraindications to vaccination are anaphylaxis to a previous dose of the same vaccine or anaphylaxis to a component of the vaccine.
Additional precautions are recommended for individuals with possible allergic reactions to a previous dose of a COVID-19 vaccine. In this instance, a specialist review by an immunology/allergy/vaccination specialist to undertake a risk/benefit assessment to assess suitability for further vaccination should be undertaken.
If an individual experiences anaphylaxis after being vaccinated with one type of COVID-19 vaccine, this does not preclude them from having another type following the additional precautions above. If there is a high risk of an allergic reaction to one of the COVID-19 vaccines due to an existing allergy to PEG or Polysorbate 80 it may be possible to a have a COVID-19 vaccine that does not contain the ingredient, depending on availability and with appropriate medical advice.
COVID-19 vaccination in people with allergic conditions
For patients with a history of anaphylaxis to food, drugs, venom or latex, it is recommended a routine observation period of 15 minutes following COVID-19 vaccination is observed.
If a patient has a history of a known systemic mast cell activation disorder with raised mast cell tryptase that has required treatment, a referral to VicSIS prior to vaccination is recommended.
Please refer to COVID-19 vaccination – ATAGI clinical guidance on COVID-19 Vaccine in Australia in 2021 or the ASCIA Allergy, Immunodeficiency, Autoimmunity and COVID-19 Vaccination Position Statement for more information.
Delayed urticaria following a COVID-19 vaccine
Acute urticaria can occur 1-2 weeks following vaccination. The symptoms can last on average 3-4 weeks but may resolve more rapidly. The urticaria can be generalised and intensely itchy. Symptoms can be managed with age-appropriate doses of non-sedating over-the-counter antihistamines (tablets or liquid) up to 4 times per day (such as cetirizine, loratadine, fexofenadine or desloratadine), best given in spaced intervals. This type of urticaria with onset that is delayed after the vaccination is generally not an indication of reproducible allergy to the vaccine and therefore investigations are not indicated. Future vaccinations can be given in a routine environment, with a 15-minute post vaccination observation period. If symptoms develop within 24 hours of vaccination, persist beyond two weeks, or there are additional concerns, then review by an immunisation specialist or allergist could be considered.
Management following anaphylaxis to a COVID-19 vaccine
Should symptoms of anaphylaxis occur immediate treatment should be provided [refer to Australian Immunisation Handbook: Adverse events following immunisation]
Investigations
Tryptase levels are recommended following potential allergic reactions as they assist with allergy assessment. Normal tryptase levels are reassuring. Tryptase levels are recommended 1 hour, 4 hours and 24 hours post reaction, however, if this is not possible, it recommended once an individual presents to an emergency department and is stable and then just prior to discharge in the case of a short admission.
It is recommended that EpiPens only be prescribed by an allergy/immunology specialist following a review. This is based on the following:
- vaccines can be easily avoided and EpiPens are on the Pharmaceutical Benefits Scheme for indications that cannot be avoided
- a prescription of an EpiPen has been shown to increase anxiety in people once it is prescribed even if it is not clinically indicated, which is thought to be due to the perceived uncertainty of future reactions.
Resources:
- Guidance for differentiating anaphylaxis and acute stress response for vaccine providers
- Journal of Allergy and Clinical Immunology: Vocal cord dysfunction/inducible laryngeal obstruction(s) mimicking anaphylaxis during SARS-CoV-2 (COVID-19) vaccination
- ASCIA Allergy, Immunodeficiency, Autoimmunity and COVID-19 Vaccination Positions Statement
- ASCIA Allergy, Immunodeficiency, Autoimmunity and COVID-19 Frequently Asked Questions (FAQ)
- ATAGI clinical guidance on COVID-19 vaccine in Australia in 2021
- MVEC: Allergy and immunisation
- MVEC: COVID-19 FAQs: allergies, pre-existing conditions and children
Authors: Rachael McGuire (MVEC Education Nurse Coordinator), Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute), Daryl Cheng (Paediatrician, The Royal Children’s Hospital), Francesca Machingaifa (MVEC Education Nurse Coordinator), Sara Barnes (Head of Allergy, Monash Health) and Adele Harris (Research Nurse, SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: November 30, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly review materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Cold chain
The cold chain describes the system of transporting and storing vaccines within the manufacturer’s recommended temperatures. It begins from when the vaccine is manufactured, transported through vaccine distribution centres, on to immunisation service providers and ends when the vaccine is administered.
Most vaccines are also sensitive to UV and fluorescent light and must be stored in their original packaging.
Failure to store and handle vaccines properly can result in reduced vaccine potency and inadequate immune responses in vaccine recipients as well as poor protection against disease.
Traditional vaccine storage
Most vaccines must be transported and stored within the traditional cold chain temperatures of between +2°C and +8°C. The optimal temperature is +5°C.
Purpose-built vaccine refrigerators are preferred for storing vaccines because they are designed and constructed specifically for vaccine storage within these temperatures. Domestic refrigerators are not suitable for vaccine storage.
For mobile or outreach immunisation clinics, vaccines can be transported in high quality coolers. Coolers must be appropriately packed using ice or gel packs and reliable temperature monitoring used throughout. Coolers are not intended for long term vaccine storage (more than 8 hours) or in extreme weather conditions.
Further information regarding traditional vaccine storage and management can be found at National Vaccine Storage Guidelines: Strive for 5.
Ultra-cold chain storage
Ultra-low temperatures are required for the storage of some COVID-19 vaccines.
Comirnaty™ (Pfizer/BioNTech) requires ultra-cold chain storage in purpose-built freezers at temperatures between -90°C and -60°C. Thermal shippers with cartons of vaccine submerged in dry ice are required for transport.
Spikevax (elasomeran) requires ultra-cold chain storage of between -25°C and -15°C. Once defrosted, Spikevax can be stored and transported at temperatures of between +2°C and +8°C for up to 30 days.
Ultra-cold chain handling equipment must be used as per manufacturer’s advice when handling frozen vaccines. Once frozen vaccines are defrosted, they cannot be refrozen.
For further information, refer to the Victorian COVID-19 vaccination guidelines.
Community pharmacy–acquired vaccines
The cold chain needs to be maintained, not only for vaccines provided as part of the National Immunisation Program but also for vaccines that a person buys from a pharmacy with a prescription. Doctors who provide a prescription for a vaccine should advise individuals that it is important to only purchase the vaccine from the pharmacy immediately before attending the practice or clinic appointment for vaccine administration. The pharmacist also has a responsibility to advise on the importance of maintaining the cold chain. On arrival to the clinic, the individual should notify reception that they have a vaccine to put in the vaccine refrigerator.
If an immunisation service provider has any concern that a vaccine provided by an individual may have been stored outside the recommended ranges, the vaccine should not be administered.
Cold chain breaches
A cold chain breach occurs when vaccines are exposed to temperatures outside the manufacturers recommended range.
A cold chain breach also includes the exposure of vaccines to light.
If a cold chain breach is suspected it is important to isolate the affected vaccines. Do not use affected vaccines until further clarification from the appropriate bodies is sought. It is important to report any cold chain breaches so that individuals can be revaccinated (if required) or unused vaccines can be recalled, if needed.
Cold chain breaches of National Immunisation Program (NIP) vaccines, influenza and travel vaccines must be reported to the Department of Health as soon as possible using the Cold Chain Breach Report form. DH will outline recommendations for the next steps to take.
Cold chain breaches of COVID-19 vaccines should be reported to the Commonwealth Vaccine Operations Centre (VOC) on 1800 318 208. The VOC will provide advice on how cold chain breaches must be managed.
Resources
- National Vaccine Storage Guidelines: Strive for 5
- Department of Health – Cold Chain Management
- Department of Health – Victorian COVID-19 vaccination guidelines
Author: Georgie Lewis (SAEFVIC Clinical Manager, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: September 2021
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Cystic Fibrosis immunisation recommendations
Background
Cystic Fibrosis (CF) is a genetic disorder, primarily affecting the lungs as well as the pancreas, liver, kidneys and intestines. In patients with CF, the cells that are responsible for producing mucous, sweat and other fluids are impaired. As a result of this, secretions become thick and sticky, impacting the function of certain organs in the body. 1 in 2500 babies in Australia are born with CF each year.
Symptoms of CF include frequent chest infections, poor weight gain and growth, intestinal obstructions, infertility (commonly in males) and diabetes. Treatment includes intensive daily physiotherapy, enzyme replacement medication, salt and vitamin supplements, exercise and a high calorie diet. Insulin and blood glucose monitoring is required for patients that develop diabetes. Many patients progress to requiring a lung transplant resulting in immunocompromise. CF patients are at high risk of infections, including some that are vaccine preventable.
Immunisation recommendations
Patients with CF are recommended to complete the routine immunisation schedule as per the National Immunisation Program (NIP) as well as some additional funded immunisations.
Secondary school vaccines are available on the NIP and should be administered to all children with CF including Year 7 (12-13 years of age): Boostrix® (diphtheria–tetanus–pertussis) and Gardasil® 9 (human papillomavirus) and in Year 10 (14-19 years) Nimenrix® (meningococcal ACWY).
Influenza
Patients with CF are funded to receive influenza vaccines annually from 6 months of age. 2 doses of the age-appropriate vaccine are required in the first year of receiving the vaccine [refer to MVEC: Influenza vaccine recommendations].
Pneumococcal
Prevenar 13® (pneumococcal conjugate) should be given 6 weeks, 4 months, 6 months (additional dose) and 12 months.
A dose of Pneumovax® 23 (pneumococcal polysaccharide) should be should be given at 4 years of age. A second dose should then be given at least 5 years later.
Further booster vaccines are required if being worked up for a lung transplant [refer to MVEC: Solid organ transplant recipient]
COVID-19
Patients with CF are at an increased risk of severe symptoms of COVID-19 disease if they become infected. A primary course of vaccination is recommended for all individuals aged 6 months and over, with booster doses recommended for some individuals, depending on age and additional risk factors.
Other vaccines to consider
Varicella
Currently a single dose of varicella vaccine is funded on the NIP. The combination MMRV (ProQuad/Priorix-tetra) is scheduled for 18-months of age, however giving 2 doses of the varicella vaccine can enhance protection. A second varicella vaccine dose can be obtained with a private script. As the varicella vaccine is a live-attenuated vaccine, doses should be separated by a minimum of 4 weeks.
Hepatitis A
CF patients are at risk of associated liver disease. The Royal Children’s Hospital funds a 2-dose course of Hepatitis A vaccines for their patient cohort. Administration can be commenced from 12 months of age.
Resources
- Cystic Fibrosis Australia
- MVEC: Solid organ transplant recipient
- Australian Immunisation Handbook: List of conditions associated with an increased risk of invasive pneumococcal disease
- DHHS: Immunisation schedule Victoria and vaccine eligibility criteria
Authors: Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute) Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Nadine Henare (Nurse Coordinator- Immunisation, The Royal Children’s Hospital)
Reviewed by: Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: August 5, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Catch-up immunisations
Where a person’s immunisations are not up to date with the National Immunisation Program (NIP), it is important to arrange a catch up schedule for immunisations to be completed in the shortest and most effective time frame to ensure protection against vaccine preventable diseases.
Prior to commencing a catch up schedule, written documentation of any previous immunisations should be obtained where possible. Documentation could include the Immunisation History Statement (AIH) from AIR, personal immunisation records (ie: green book) or medical records. Overseas records may need translating.
Serology is not routinely recommended prior to catch up immunisation.
All refugee and humanitarian entrants including asylum seekers are eligible for free catch up immunisations [see resources].
Please be aware that some individuals may be recommended to receive additional immunisations due to predisposing conditions or at risk circumstances. For further queries please contact MVEC here.
For individuals <10 years of age
Australian Immunisation Handbook: catch up calculator
For individuals aged 10 years to 19 years
Victorian immunisation catch-up tool
Refugee and asylum seekers
The Victorian DHHS and Health Translations has an excellent resource for catch up immunisations in refugees and asylum seekers. It has been translated into more than 10 languages (see Catch up vaccinations for refugees and asylum seekers in Victoria).
Resources
- DHHS: Victorian immunisation schedule and eligibility criteria for free vaccines
- Vaccine history timeline in Australia
- Australian Immunisation Handbook: Catch up vaccination
- Victorian Refugee Health Network
- Vaccine schedule by country
Authors: Nigel Crawford (Paediatrician, The Royal Children’s Hospital, Melbourne) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: January 31, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Children with Cardiac Disease
Background
Childhood cardiac disease can be congenital or acquired. Congenital cardiac disease describes a number of conditions including malformations of the heart, major blood vessels or heart valves. Approximately 1 in 100 babies born in Australia each year will have some form of congenital heart disease. Acquired cardiac disease can include rheumatic heart disease or cardiac disease secondary to Kawasaki disease.
Children with underlying cardiac disease are at increased risk of complications of vaccine preventable diseases when compared to children who do not have cardiac disease. This can be exacerbated by an increased risk of exposure due to the need for frequent health care appointments and hospital stays. Those at highest risk include children with cyanotic heart disease or cardiac failure.
Vaccine recommendations
Children with cardiac disease can safely receive vaccines according to the National Immunisation Program (NIP) vaccine schedule. Additional vaccines such as pneumococcal, influenza and COVID-19 vaccines are also recommended (see Table 1).
Family members and household contacts are recommended to be up to date with all vaccines including pertussis, annual influenza and COVID-19 vaccines. Close contacts of children with cardiac disease can receive live-attenuated vaccines without the need for additional precautions.
Table 1: Vaccine recommendations for children with cardiac disease
| Vaccine | Recommendation |
|---|---|
| Influenza | Annual influenza vaccines are recommended from 6 months of age. Two doses of age-appropriate vaccines are required in the first year of vaccination for children < 9 years. |
| Pneumococcal | An additional pneumococcal conjugate vaccine (Prevenar 13®) is recommended at 6 months of age (or at diagnosis, whichever is later). A pneumococcal polysaccharide vaccine (Pneumovax 23®) should be given at 4 years of age (minimum 8 weeks post Prevenar13, whichever is later), followed by another dose at least 5 years later (max. 2 doses in a lifetime). |
| COVID-19 | Children with cardiac disease are at a greater risk of severe COVID disease. A primary course of COVID-19 vaccines are recommended for children aged 6 months and above with further booster doses recommended for those aged ≥ 12 years. |
| Travel vaccines | Specialist travel advice should be sought when travelling overseas, particularly to high risk areas for vaccine preventable diseases. |
Children with cardiac disease who are to undergo a transplant should also receive further vaccines as part of their workup prior to transplant.
Immunisation queries should be directed to the treating doctor and/or the Royal Children’s Hospital Immunisation Service.
Vaccine precautions
Precautions are recommended when vaccinating children with cardiac disease in the following circumstances:
- in children who are immunocompromised, live-attenuated vaccines may be contraindicated
- children who have received blood products/and or immunoglobulin may need to delay vaccination
- if children are also asplenic or have hyposplenism, further additional vaccines are recommended
- children for whom cardiac surgery is indicated:
- before surgery – inactivated vaccines can be administered up until 1 week prior to surgery, live-attenuated vaccines can be administered up until 3 weeks prior to surgery (e.g. MMR, varicella)
- vaccines indicated following surgery should be delayed for at least one week due to the potential for confusing expected vaccine side effects with post-operative complications.
Resources
- MVEC: Influenza vaccine recommendations
- MVEC: COVID-19 vaccination in children and adolescents
- MVEC: Pneumococcal
- MVEC: Needle phobia
- Department of Health: Immunisation for people with medical risk conditions
- Heart Kids: Congenital heart disease and COVID-19
Author: Kirsten Mitchell (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: August 5, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy. You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.