Mpox (monkeypox)
What is it?
Mpox is a viral zoonosis (an infection spread from animals to humans). It is caused by a virus that belongs to the Orthopoxvirus genus (which also causes the variola virus responsible for smallpox disease and the vaccinia virus, which is used in smallpox vaccines). Mpox was first discovered in 1958 and there have been small outbreaks since, predominantly in Western and Central Africa. Since the eradication of smallpox in 1980, mpox has become the most important orthopoxvirus affecting humans, however, causes less severe disease than smallpox.
What to look for
The incubation period of mpox is usually 7-14 days, but can be as short as 5 days or as long as 21 days. The initial symptoms include fever, headache, backache and muscle aches, fatigue and lymphadenopathy. Lymphadenopathy in this early phase is a key feature of mpox.
1-3 days following the beginning of fever, a rash may develop, often beginning on the mouth and face, and then spreading to other areas of the body. The face is involved in 95% infections, followed by the palms of the hands and soles of the feed (75%). Oral mucous membranes are involved in 70% of cases, and involvement of the genitalia is also common (30%).
The rash is initially characterised as being erythematous (reddened) and macular (flat), which then develops papular features (raised areas) and turns into well-demarcated pustules and vesicles. These then dry into crusts and fall off. The number of lesions is highly variable, ranging from a couple to over a thousand.
The infection is usually self-limiting with symptoms lasting from 2-4 weeks. Complications can include secondary infection such as cellulitis, sepsis, infection of the cornea (which can be threaten vision), bronchopneumonia and encephalitis. The case fatality ratio is between 3-6%. More severe illness can occur in immunocompromised people.
How is it transmitted?
Mpox is spread via either animal-to-human transmission (zoonotic) or human-to-human transmission.
Zoonotic transmission involves direct contact with the bodily fluids, blood, or lesions (cutaneous or mucosal) of infected animals. This is most common for people living near or within forest areas with exposure to infected animals.
Human-to-human transmission involves close contact with respiratory secretions, the skin lesions of an infected person, or a contaminated objects such as linen or clothing. Droplet particle transmission requires prolonged face-to-face exposure, placing household contacts at highest risk. This can be minimised by isolating away from other members of the household.
Healthcare workers caring for individuals with mpox must undertake infection control precautions and handling of laboratory specimens should be by suitably trained staff.
Epidemiology
Mpox is very common in West and Central Africa, often in areas with tropical rainforests. However, there are currently outbreaks in many other countries across the globe including Australia and parts of Europe and the United Kingdom.
Vaccines
The vaccinia virus is a poxvirus related to smallpox and mpox and is contained in smallpox vaccines. Historically, smallpox vaccines have been used in the prevention of smallpox infection, however, they are also likely to be effective against mpox.
There are two types of smallpox vaccines available for use in Australia for the prevention of mpox:
- ACAM2000™ – 2nd generation, live-attenuated vaccine
- JYNNEOS® – 3rd generation, non-replicating vaccine
Recommendations
Either vaccine can be administered subcutaneously as either primary preventative vaccination (PPV) or post-exposure preventative vaccination (PEPV) based on an individual risk-benefit assessment. ATAGI preferentially recommends JYNNEOS® vaccine due to the ease of administration and decreased likelihood of side effects.
For PPV, administering JYNNEOS® via the intradermal route can be considered in all eligible populations to maximise vaccine supply. In circumstances where vaccine supply is limited severely immunocompromised individuals should be prioritised over other eligible groups such as healthcare workers to receive their second dose of JYNNEOS® (ACAM2000™ is contraindicated in this population). Intradermal vaccination is currently recommended for PPV only.
For PEPV, vaccination within 4 days is recommended to provide optimal protection against the development of mpox infection. Vaccination between 4-14 days following exposure may lessen the severity of disease.
Vaccination is currently recommended for the following groups:
- those who are categorised by public health authorities as a high-risk mpox contact in the previous 14 days (PEPV)
- gay, bisexual, and other men, non-binary individuals assigned male at birth, trans men who have sex with men (including cis and trans men) who are the highest risk of infection with mpox. Markers indicating increased risk of infection include:
- those living with HIV
- recent history of multiple sexual partners, including group sex or sex on licensed premises
- other markers including recent sexually transmitted disease or on HIV PrEP due to number of partners
- recommendations from sexual health clinics
- sex workers, particularly those with clients who belong to high-risk groups
- anyone in the above categories planning to travel to a country experiencing a mpox outbreak (immunisation is recommended 4-6 weeks prior to travelling)
- anyone at greater risk of poorer outcomes due to mpox infection, such as those with severe immunocompromise
- immunisation providers who are administering ACAM2000™.
Individuals who have a history of confirmed mpox infection should defer vaccination in the short-medium term after recovery due to the immunity gained from natural infection.
Precautions
Vaccination with JYNNEOS® can be considered in children aged < 18 years where the benefits of vaccination outweigh the risks of disease. Despite limited safety data, there are no theoretical safety concerns surrounding the administration of JYNNEOS® in pregnant or breastfeeding women.
Individuals receiving ACAM2000™ as PPV should consider an interval of 4 weeks between vaccination and administration of COVID-19 vaccines due to the rare risk of myocarditis/pericarditis.
Individuals with a history of keloid scarring are not recommended to receive JYNNEOS® via the intradermal route, with subcutaneous being preferred.
Contraindications
Anyone with a history of anaphylaxis to a previous dose of the vaccine to be administered or anaphylaxis to a component of the vaccine to be administered should not be vaccinated. Due to the risk of vaccine associated disease, ACAM2000™ is contraindicated for those with immunocompromise or those who are pregnant.
Individuals with active eczema, atopic dermatitis or other exfoliative skin conditions should not receive ACAM2000™ due to the risk of developing eczema vaccinatum (a reaction to smallpox vaccination experienced by people with eczema/atopic dermatitis resulting in a severe rash and systemic illness).
| Vaccine | Age | Platform | Route | Volume | Primary course | Booster |
| JYNNEOS® | ≥ 18 years | Non-replicating ankara vector | Subcutaneous § | 0.5ml | 2 doses, min. 28 days apart* | If previous smallpox vaccine was received > 10 years ago |
| Intradermal §€£ | 0.1ml | 2 doses, min. 28 days apart | ||||
| ACAM2000™ ¥ | ≥ 18 years | Live-attenuated | Percutaneous ^# | 1 droplet (0.0025 mL) of reconstituted vaccine | 1 dose |
*an interval longer than 28 days is acceptable. If supply is limited, severely immunocompromised individuals should be prioritised to receive their 2nd dose as close to 28 days from dose 1 as possible.
§ Using alternate routes of vaccine delivering to complete a primary course is acceptable (eg, intradermal for dose 1 and subcutaneous for dose 2)
€ Intradermal administration is an alternate route of vaccination for pre-exposure prophylaxis. It is not preferred for the first dose of post-exposure prophylaxis and is NOT recommended in people with severe immunocompromise
£ Vaccination providers administering intradermally should ensure that they are appropriately trained in intradermal technique. Where a dose of intradermal vaccine is inadvertently administered subcutaneously, a repeat dose of 0.5ml should be administered subcutaneously as soon as possible to ensure that the vaccinee receives an appropriate level of protection
^ Percutaneous administration involves using a bifurcated needle and scarification technique requiring specialised training and accreditation
# for full aftercare instructions, refer the product information
¥ ACAM2000 is a live-attenuated vaccine and therefore as outlined above, it’s use is contraindicated in some patient groups. For a full list of contraindications and precautions refer to the ATAGI clinical guidance on vaccination against Mpox.
Following vaccination
Individuals receiving ACAM2000™ should be advised that 2-5 days after vaccination a papule will form at the injection site. This will progress to a vesicle (blister) then pustule (blister with pus) before scabbing and forming a permanent pitted scar. Individuals are recommended to cover the injection site with a gauze bandage secured with adhesive tape until scabbing occurs, noting that the wound is infectious until the wound dries up.
Common systemic side effects following vaccination with either vaccine include muscle aches, headache, fatigue and nausea. Localised side effects can include injection site itch, pain redness and swelling (and permanent scarring following ACAM2000™.
ACAM2000™ is also associated with a risk of myocarditis and pericarditis as well as other serious side effects noted here.
Resources
Victorian program information
Other resources
- ATAGI clinical guidance on vaccination against mpox
- Australian Government Department of Health and Aged Care: Mpox resources
- Mpox virus infection: CDNA National Guidelines for Public Health Units
- Better Health Channel: Mpox
Authors: Rachael Purcell (RCH Immunisation Fellow), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: March 23, 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Mandatory vaccination
Background
A vaccine mandate means that proof of vaccination, or an authorised medical exemption, is required in certain settings. Mandating is generally considered a late step in optimising vaccine uptake, as it is important to ensure vaccine access is available in an equitable way, before progressing to a mandate. Vaccine mandates may vary depending on the jurisdiction and there may be some variations around Australia. The following information is specific to Victoria, and refers to COVID-19 and influenza vaccine directions, as well as the ‘no jab, no play’ and ‘no jab, no pay’ policies.
Vaccine specific directions
COVID-19
Vaccination against COVID-19 protects against developing severe disease that may lead to hospital admission, intensive care or even death. Due to the occupational risk for disease exposure and transmission as well as having increased contact with vulnerable groups, workers in the following settings must be fully vaccinated against COVID-19 (regardless of whether they have patient contact or not):
- public, private and denominational hospitals
- public health services
- private day procedure centres
- ambulance services
- patient transport services engaged by a health service or Ambulance Victoria
- residential aged care services that are operated by public health services.
To be considered fully vaccinated, individuals ≥ 18 years must have completed a primary schedule of COVID-19 vaccination plus a booster dose and those aged < 18 years must have completed a primary schedule.
For further information refer to DH: Vaccination for healthcare workers.
Influenza
Influenza vaccination is recommended for all individuals aged 6 months and over. Due to an increased risk of exposure to, and transmission of influenza disease it is mandatory that workers in the following settings are vaccinated against influenza annually (regardless of whether they have patient contact or not):
- public, private and denominational hospitals
- public health services
- private day procedure centres
- ambulance services
- patient transport services engaged by a health service or Ambulance Victoria
- residential aged care services that are operated by public health services
- Forensicare.
For further information, refer to DH: Vaccination for healthcare workers.
Policy specific vaccine directions
No jab, no play
“No jab, no play” legislation was introduced by the Victorian State Government on January 1st, 2016, in an effort to improve vaccination rates and reduce the spread of vaccine preventable diseases, This legislation requires confirmation of up to date vaccination status according to the National Immunisation Program (NIP) when enrolling in all early childhood education and care services including childcare and kindergarten. This legislation does not apply to enrolment into school.
A current Immunisation History Statement (IHS) provided by the Australian Immunisation Register (AIR) is the only accepted proof of immunisation when enrolling in early childhood education and care services.
No jab, no pay
“No jab, no pay” legislation was introduced by the Federal Government on July 1, 2018, altering Family Tax Benefit (FTB) Part A payments and childcare subsidies if a child is not up to date for age with their scheduled immunisations as per the NIP. Recipients of FTB part A or child-care fee assistance will need to meet these immunisation requirements to ensure that payments are not reduced.
Responsibilities of the employer/service provider and the employee/vaccinee
Employers and service providers are responsible for ensuring that employees/enrolees in early childhood education and care services comply with relevant orders. The employer or service provider is required to sight and store evidence of mandated vaccination status or medical exemption, if applicable.
It is the responsibility of the employee to be vaccinated against mandated vaccines for their profession or have a valid medical exemption.
It is the responsibility of individual receiving Family Tax Benefits and childcare subsidies to ensure that children are up to date with the NIP.
Medical exemptions
Exemption to vaccination can be granted to individuals following assessment by an authorised practitioner who deems deferral of vaccination to be warranted. Exemptions may be permanent or temporary.
All medical exemptions to vaccination will be recorded on the individuals Immunisation History Statement on the Australian Immunisation Register which can be accessed via myGOV.
Individuals who have previously experienced or who are at higher risk of experiencing a serious adverse event following vaccination should be referred to a specialist immunisation service. Individuals who have experienced a previous adverse event following vaccination should also be reported to SAEFVIC.
Permanent medical exemptions
Anaphylaxis to a previous dose of the same vaccine or anaphylaxis to a component of the same vaccine are the only two absolute contraindications to vaccination and warrant permanent medical exemption. Permanent exemptions to some routine vaccines can also be provided when an individual has documented evidence of natural immunity against that vaccine preventable disease (eg. varicella, measles-mumps-rubella or hepatitis B). In addition, there are a small group of specific medical conditions precluding some individuals from receiving certain COVID-19 vaccine brands.
Temporary medical exemptions
Temporary exemptions to vaccination can also be granted in circumstances such as acute major medical illness, significant immunocompromise of short duration (for live-attenuated vaccines only), the individual is pregnant (live-attenuated vaccines only). Individuals with a confirmed history of COVID-19 infection can have their COVID-19 vaccination deferred for up to 4 months).
Children on an approved catch-up schedule will be automatically granted a grace period of 6 months to complete their outstanding vaccines and be up to date with the NIP.
Authorising providers
General practitioners defined by the Health Insurance Act 1973 as:
- fellows of the Royal Australian College of General Practitioners
- fellows of the Australian College of Rural and Remote Medicine
- on Medicare’s Vocation Register of General Practitioners
or:
- a practice registrar on an approved 3GA training placement
- a paediatrician
- a public health physician
- an infectious diseases physician
- a clinical immunologist.
Resources
General
- DH: Vaccination for healthcare workers
- Services Australia: Record a medical contraindication
- Services Australia: Australian Immunisation Register (AIR) – immunisation medical exemption form (IM011)
COVID-19 and influenza
- DH: Vaccination for healthcare workers
- ATAGI Clinical guidance for COVID-19 vaccine providers
- ATAGI Expanded Guidance on temporary medical exemptions for COVID-19 vaccines
- Discussion guide for medical exemptions
- Health Services Amendment (Mandatory Vaccination of Healthcare Workers) Act 2020
No jab no play/No jab no pay
- DH: No Jab No Play – Resources for providers
- DH: No Jab No Play
- Better Health Channel – No Jab No Play
- DH: Immunisation schedule Victoria and vaccine eligibility criteria
Authors: Rachael McGuire (MVEC Education Nurse Coordinator) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: April 4, 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Meningococcal
What is it?
Meningococcal disease constitutes any illness caused by the bacteria Neisseria meningitidis. There are 13 known sub-types (serogroups) and of these, 5 are currently vaccine preventable (B and A, C, W, Y).
Invasive meningococcal disease (IMD) can cause meningitis (inflammation of the membrane covering the brain and spinal cord) and septicaemia (infection in the blood), as well as other infections like pneumonia (lung infection), arthritis (inflammation of the joints) and conjunctivitis (eye infection). Mortality (death) can be as high as 5-10% with permanent lifelong complications occurring in 10-20% of those who survive.
What to look for
The incubation period of meningococcal is 1-7 days, more commonly 3-4 days. People with meningococcal disease can become extremely unwell very quickly. Symptoms can include fever, headache, neck stiffness, nausea, vomiting and photophobia (sensitivity to light). Cool, mottled extremities and leg pain can also occur. Babies can appear irritable or unsettled, have a high-pitched moaning cry, refuse or not wake for feeds and be lethargic (sleepy) or floppy. A petechial or purpuric rash can appear late in the disease progression (within 13-22 hours) or not at all.
How is it transmitted?
Disease can be transmitted from person to person via respiratory droplets (eg. sneezing and coughing). Meningococcal bacteria can also live harmlessly at the back of the nose or throat, resulting in individuals being asymptomatic carriers.
Epidemiology
Children < 2 years of age have the highest incidence of meningococcal disease in Australia, with another peak of disease among adolescents and young adults (15-24 years). Aboriginal and Torres Strait Islander people have a much greater burden of disease than non-Indigenous people.
There are also certain medical conditions and medications that can increase an individual’s risk of IMD. These include (but are not limited to) those with functional asplenia and hyposplenia, complement deficiency and those receiving treatment with eculizamab [see below for specific information for those with increased risk of IMD].
Prevention
MVEC strongly recommends everyone wishing to be protected against ACWY and B strains of meningococcal disease be immunised. Some individuals are eligible for funded vaccines via the National Immunisation Program (NIP). Those aged ≥ 6 weeks of age who do not meet the funding criteria can purchase vaccines privately through some councils, GPs and pharmacies.
The number of vaccine doses recommended depends on a person’s age and risk factors for IMD.
Meningococcal ACWY vaccines
MVEC recommends 2 conjugate meningococcal ACWY vaccines:
- Nimenrix®
- Menveo®
A single dose of Nimenrix® is currently provided for free at 12 months of age and for all adolescents in Year 10 (or age equivalent) with catch up available for those aged 15-19 years. It is also funded for certain individuals of any age with immunocompromising conditions.
Meningococcal ACWY primary course and booster doses for healthy individuals
Primary course
WordPress Tables PluginVaccine brand Course commenced at 6 weeks to ≤ 5 months of age Course commenced at 6 months to ≤ 11 months of age Course commenced at 12 months to ≤ 23 months of age Course commenced at ≥ 2 years of age Nimenrix®† 3 doses (minimum 8 weeks between 1st and 2nd doses; 3rd dose given at ≥ 12 months of age/8 weeks after 2nd dose, whichever is later)¥ 2 doses (2nd dose at ≥ 12 months of age/8 weeks after 1st dose, whichever is later)¥ 1 dose¥ 1 dose Menveo®† 3 doses (minimum 8 weeks between 1st and 2nd doses; 3rd dose given at ≥ 12 months of age/8 weeks after 2nd dose, whichever is later)¥ 2 doses (2nd dose at ≥ 12 months of age/8 weeks after 1st dose, whichever is later)¥ 2 doses (minimum 8 weeks apart)¥ 1 dose † there is no registered upper age limit for the use of Menveo® or Nimenrix®.
¥ completing the course with the same vaccine brand is preferred but may not always be practical. The NIP funded 12 month dose of Nimenrix® may be used as the dose given at ≥ 12 months of age.Booster doses
Further booster doses are not routinely recommended for healthy individuals. In circumstances where someone has previously received a primary course of meningococcal ACWY and is offered a further dose in year 10 in line with the NIP, it is acceptable to to receive this dose.
Meningococcal ACWY primary course and booster doses for those at increased risk of IMD
Individuals with specified medical conditions that increase the risk of IMD are recommended and funded to receive additional meningococcal vaccines and booster doses. These groups include:
- those with defects in, or deficiency of complement components (including factor H, factor D or properdin deficiency),
- those currently receiving or planning treatment with eculizumab (or biosimilar),
- those with functional or anatomical asplenia (including sickle cell disease or haemoglobinopathies and congenital or acquired asplenia),
- anyone with HIV (regardless of disease stage or CD4+ cell count),
- anyone who previously received a haemopoietic stem cell transplant (HSCT).
Primary course
WordPress Tables PluginVaccine brand Course commenced at 6 weeks to ≤ 5 months of age Course commenced at 6 months to ≤ 11 months of age Course commenced at ≥ 12 months of age Nimenrix®† 4 doses (minimum 8 weeks apart, with the 4th dose given at ≥ 12 months of age/more than 8 weeks after the 3rd dose, whichever is later)¥ 3 doses (minimum 8 weeks apart, with the 3rd dose given at ≥ 12 months of age/8 weeks after 2nd dose, whichever is later)¥ 2 doses (minimum 8 weeks apart)¥ Menveo®† 4 doses (minimum 8 weeks apart, with the 4th dose given at ≥ 12 months of age/more than 8 weeks after the 3rd dose, whichever is later)¥ 3 doses (minimum 8 weeks apart, with the 3rd dose given at ≥ 12 months of age/8 weeks after 2nd dose, whichever is later)¥ 2 doses (minimum 8 weeks apart)¥ † there is no registered upper age limit for the use of Menveo® or Nimenrix®.
¥ completing the course with the same vaccine brand is preferred but may not always be practical. The NIP funded 12 month dose of Nimenrix® may be used as the booster dose for those who have commenced the course at < 12 months of age.Booster doses
WordPress Tables PluginVaccine brand Where the primary course was completed at ≤ 6 years of age Where the primary course was completed at ≥ 7 years of age Nimenrix®†§ OR Menveo®†§ Give a booster dose 3 years following the completion of the primary course, then further booster doses every 5 years Give a booster dose every 5 years following the completion of the primary course † there is no registered upper age limit for the use of Menveo® or Nimenrix®.
§ using either Menveo® or Nimenrix® as a booster dose, regardless of the brand used for the primary course, is approriate.
Meningococcal B vaccines
There are currently 2 vaccines available for protection against meningococcal B disease.
- Bexsero®
- Trumenba®
Meningococcal B vaccines brands are not interchangeable.
A primary course of Bexsero® is available on the NIP for Aboriginal and Torres Strait Islander children < 2 years of age, as well as some individuals of any age with immunocompromising conditions.
Paracetamol advice
It is widely recognised that children receiving Bexsero® are more likely to experience fever following vaccination. It is for this reason that children < 4 years of age are recommended to receive prophylactic paracetamol (15mg/kg per dose) 30 minutes prior to vaccination (or as soon as possible after), as well as 2 subsequent doses (4-6 hours apart) to reduce the likelihood and severity of fever. This should be administered regardless of whether the child is experiencing a fever or not.
Meningococcal B primary course and booster doses for healthy individuals
Primary course
WordPress Tables PluginVaccine brand Course commenced at 6 weeks to ≤ 11 months of age Course commenced at 12 months to ≤ 9 years of age Course commenced at ≥ 10 years Bexsero®† 3 doses (minimum 8 weeks between 1st and 2nd doses; 3rd dose at ≥ 12 months of age/more than 8 weeks after the 2nd dose, whichever is later)¥#£ 2 doses (minimum 8 weeks apart)¥#£ 2 doses (minimum 8 weeks apart)¥ Trumenba®† N/A N/A 2 doses (minimum 6 months apart)¥ † Bexsero® is registered for use in those 6 weeks of age and older. Trumenba® is only registered for use in those 10 years of age or older.
¥ meningococcal B vaccine brands are not interchangeable for primary courses or booster doses.
# prophylactic paracetamol is recommended to those < 4 years of age (refer to advice above).
£ funded on the NIP for Aboriginal and Torres Strait Islander children < 2 years of age and those identified as medically at risk (see recommendations below for further information).
N/A- not recommended in this age group.Booster doses
Further booster doses of meningococcal B vaccines are not routinely recommended for healthy individuals.
Meningococcal B primary course and booster doses for those with increased risk of IMD
Individuals with specified medical conditions that increase the risk of IMD are recommended and funded to receive additional meningococcal B vaccines. From December 2022, following an NCIRS-led GRADE review of the evidence, ATAGI endorsed an update to the Australian Immunisation Handbook recommendations which now include booster doses of meningococcal B vaccines.
Eligible individuals include:
- those with defects in, or deficiency of complement components (including factor H, factor D or properdin deficiency),
- those currently receiving or planning treatment with eculizumab (or biosimilar),
- those with functional or anatomical asplenia (including sickle cell disease or haemoglobinopathies and congenital or acquired asplenia),
- anyone with HIV (regardless of disease stage or CD4+ cell count),
- anyone who previously received a haemopoietic stem cell transplant (HSCT).
MVEC strongly encourages the active follow up of individuals who meet these criteria to ensure that appropriate vaccine schedules and their recommended booster doses are administered in line with the updated guidance to optimally protect vulnerable patients.
Primary course
WordPress Tables PluginVaccine brand Course commenced at 6 weeks to ≤ 5 months of age Course commenced at 6 months to ≤ 11 months of age Course commenced at 12 months to ≤ 9 years of age Course commenced at ≥ 10 years of age Bexsero®† 4 doses (minimum 8 weeks apart, with the 4th dose given at ≥ 12 months of age/more than 8 weeks after the 3rd dose, whichever is later)¥# 3 doses (minimum 8 weeks apart, with the 3rd dose given at ≥ 12 months of age/8 weeks after 2nd dose, whichever is later)¥# 2 doses (minimum 8 weeks apart)¥# 2 doses (minimum 8 weeks apart)¥ Trumenba®† N/A N/A N/A 3 doses (1 months between 1st and 2nd doses; 6 months between 1st and 3rd doses)¥ † Bexsero® is registered for use in those 6 weeks of age and older. Trumenba® is only registered for use in those 10 years of age or older.
¥ meningococcal B vaccine brands are not equivalent or interchangeable for primary courses or booster doses.
# prophylactic paracetamol is recommended to those < 4 years of age (refer to advice above).
N/A- not recommended in this age group.Booster doses§
WordPress Tables PluginVaccine brand Where the primary course was completed at ≤ 6 years of age Where the primary course was completed at ≥ 7 years to ≤ 9 years of age Where the primary course was completed at ≥ 7 years of age Bexsero®† Give a single booster dose 3 years after completing the primary course¥ Give a single booster dose 5 years after completing the primary course¥ Give a single booster dose 5 years after completing the primary course¥ Trumenba®† N/A N/A Give a single booster dose 5 years after completing the primary course¥ § refer to GRADE assessment for more information.
† Bexsero® is registered for use in those 6 weeks of age and older. Trumenba® is only registered for use in those 10 years of age or older.
¥ meningococcal B vaccine brands are not equivalent or interchangeable for primary courses or booster doses.
N/A- not recommended in this age group.
Resources
- Better Health Channel: Meningococcal disease- immunisation
- Australian Immunisation Handbook: Meningococcal
- RCH Kids Health information: Meningococcal infection
- ATAGI clinical advice on changes to recommendations for meningococcal vaccines from 1 July 2020
- MVEC: Febrile seizures (febrile convulsions) and vaccines
- MVEC: Asplenia and hyposplenia
- NCIRS: Meningococcal vaccines GRADE assessments
Authors: Rachael McGuire (MVEC Education Nurse Coordinator), Georgina Lewis (Clinical Nurse Manager, SAEFVIC, Murdoch Children’s Research Institute) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Date: July 4, 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Myocarditis and pericarditis following COVID-19 vaccines
Myocarditis is an inflammatory disease of the heart muscle, whilst pericarditis is an inflammatory disease of the lining of the heart muscle. They are rare conditions, most commonly associated with viral infections (including SARS-CoV-2) but can also be triggered by other factors such as medications and autoimmune conditions.
Globally, an increased number of cases above an expected population rate of myocarditis and pericarditis have been reported in individuals who have received COVID-19 vaccines, with the highest rates occurring following administration of COVID-19 mRNA vaccines (eg. Comirnaty (Pfizer) and Spikevax (Moderna)).
Information specific to myocarditis and pericarditis can be found via the below buttons. In addition, frequently asked questions relating to vaccination are also addressed.
How is myocarditis following COVID-19 vaccination triggered?
The exact mechanism behind cardiac inflammation temporally associated with COVID-19 vaccines is currently being investigated. Clinical causes from international and local surveillance data suggest an immune-mediated or hypersensitivity trigger. There are ongoing studies examining the role of the SARS-CoV-2 spike protein, impact of certain cardiac biomarkers and genetic predispositions to this adverse event of special interest (AESI).
Who is at risk of myocarditis?
Myocarditis from any cause, occurs more commonly in males than females. It is also more likely to affect younger adults.
Reported rates of myocarditis occurring following administration of COVID-19 vaccines vary; however, they are above expected background rates for both sexes. The peak risk group for COVID-19 vaccine related myocarditis is young adult males aged 16-17 years, with a smaller increased risk for males aged between 12-24 years.
International and local vaccine safety surveillance data have found that it is more commonly associated with administration of a second dose of COVID-19 mRNA vaccine. Myocarditis following other COVID-19 vaccines, third doses or booster doses have also been identified, although reported cases have occurred at lower rates than those identified following either dose of a primary course.
Although myocarditis AESI has been associated with all of the COVID-19 vaccines used in Australia, there is a higher risk of myocarditis following administration of COVID-19 mRNA vaccines compared to non-mRNA vaccines. Surveillance data from multiple countries have also demonstrated a greater risk with Spikevax (Moderna) compared to Comirnaty (Pfizer).
Are children more likely to experience myocarditis following COVID-19 vaccination?
Available safety data from local and international sources suggest there is a significantly lower risk of children developing myocarditis following vaccination. Thus far, data shows that the risk of COVID-19 vaccine myocarditis decreases as age decreases.
ATAGI recommends an interval of 8 weeks between vaccine doses in a primary course for age-eligible children (≤ 11 years) for maximum efficacy and safety. This extended interval is based on international data suggesting a longer time interval between dose 1 and 2 may reduce the risk of myocarditis. This extended interval also allows more time to observe international vaccine safety data and identify any signals for rare adverse events.
Very few cases of myocarditis in children <6 years of age have been reported in available worldwide surveillance data to date.
For more information on COVID-19 vaccination in individuals < 18 years please refer to COVID-19 vaccination in children and adolescents.
Pre-existing cardiac conditions and COVID-19 vaccination
Individuals with the following cardiac conditions can safely receive COVID-19 vaccines without the need for additional monitoring or precautions:
- coronary artery disease
- myocardial infarction
- stable heart failure
- arrhythmias
- rheumatic fever
- rheumatic heart disease
- kawasaki disease
- most congenital heart disease
- those with implanted cardiac devices
- congenital heart disease
- cardiac transplant
- cardiomyopathy.
Those with a history of the following conditions can also receive COVID-19 vaccines; however should consult their treating specialist to determine the appropriate timing for vaccination:
- recent (within 3 months) or current inflammatory cardiac conditions (including myocarditis, pericarditis and endocarditis)
- acute rheumatic fever or acute rheumatic heart disease
- acute decompensated heart failure.
Patients with ongoing cardiac inflammation should have vaccination deferred. In some instances, vaccination with Vaxzevria (AstraZeneca) or Nuvaxovid (Novavax) may be considered due to the lower associated risk of developing myocarditis.
What are the symptoms of myocarditis?
Myocarditis presents similarly to pericarditis, with a range of symptoms including:
- chest pain, pressure or discomfort
- pain with breathing (pleuritic chest pain)
- shortness of breath
- palpitations
- syncope (faint)
- other non-specific symptoms such as fatigue, dizziness, abdominal pain.
In individuals who have received COVID-19 vaccines, symptoms of myocarditis have most commonly been reported within 2-7 days of second dose vaccination.
How is myocarditis after COVID-19 vaccination diagnosed and investigated?
If there is suspicion of myocarditis, particularly in the first week following vaccination, timely medical review is important. Those who appear unwell should be referred to an emergency department for examination and the following primary investigations:
- blood tests for cardiac biomarkers, such as troponin
- electrocardiogram (ECG).
Other tests should be considered if the patient is unwell or the tests above are abnormal:
- chest X-ray (CXR)
- other tests related to investigating differential diagnoses such as inflammatory markers (C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)
- bedside or formal ultrasound/echo
- cardiac MRI.
Consistent findings of myocarditis may include elevated troponin, ECG changes (ST or T-wave abnormalities, premature atrial or ventricular complexes) as well as abnormal echocardiogram or cardiac MRI.
Individuals who are investigated for myocarditis following vaccination should avoid high-intensity exercise until symptoms have resolved is recommended.
Cardiologist advice and followup is strongly recommended.
How is myocarditis after COVID-19 vaccination treated?
Current data shows that most cases of myocarditis following COVID-19 vaccination have mild symptoms and recover well. Information on long term sequelae is not yet available.
Treatment of these conditions is managed by a cardiologist and include in-patient supportive therapies. In the rare severe or complicated cases, specific management for arrythmias, decreased cardiac function or congestive cardiac failure with pharmacological agents such as ACE-inhibitors and beta-blockers or mechanical support may be necessary.
I have specific questions around my risk of myocarditis following COVID-19 vaccination. Help!
Although uncommon, myocarditis is most often seen after COVID-19 mRNA vaccines. Please refer to our specific FAQs on COVID-19 mRNA vaccines for further information. This includes answers on:
- impact of dose intervals between 1st and 2nd doses of vaccine
- risk of developing myocarditis from COVID-19 disease vs the vaccine
- exercise after COVID-19 vaccination
- impacts of medications and drugs such as clozapine, stimulants, amphetamines on developing myocarditis.
What are the implications for future doses (including third and booster doses)?
For individuals where the cause of inflammation is attributed to COVID-19 vaccination, a report to SAEFVIC is indicated and a referral to a cardiologist and/or specialist immunisation service (eg. VicSIS) is recommended.
Please refer to the below algorithm for recommendations relating to further doses of COVID-19 vaccines in patients who have been diagnosed with myocarditis following vaccination.
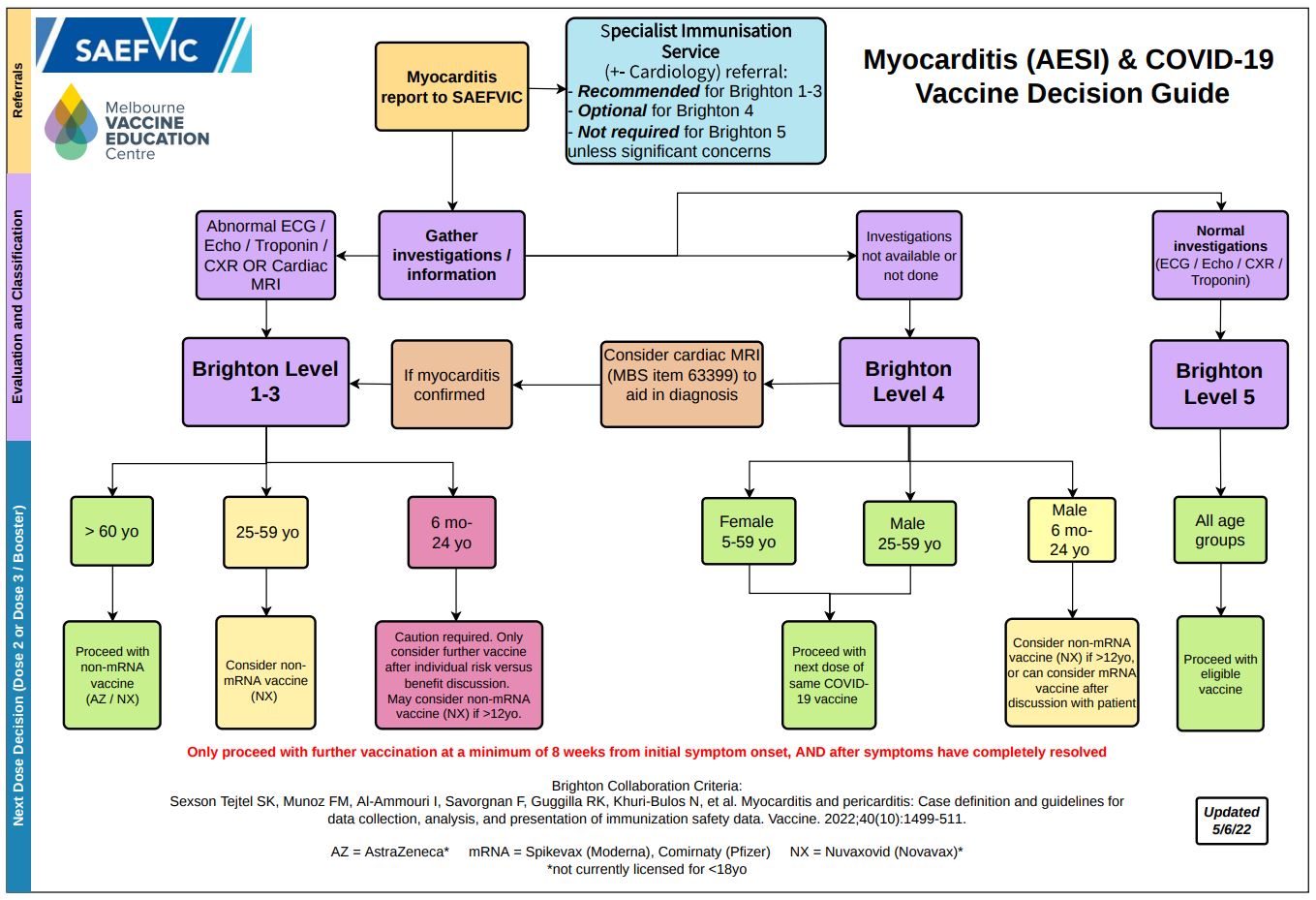 *Brighton Level refers to Brighton Collaboration criteria for classifying myocarditis
*Brighton Level refers to Brighton Collaboration criteria for classifying myocarditis
PDF version of the above diagram available here.ATAGI currently recommends that individuals diagnosed with myocarditis following vaccination defer any further doses and are referred to a specialist immunisation clinic.
What is the risk/benefit ratio for vaccination for the young adult age group?
It is important to discuss your individual circumstances with a health care provider in order to make an informed decision.
The level of COVID-19 community transmission in Australia can change quickly. Factors to consider include age, potential for exposure to the virus (including in the workplace), high rates of global transmission, the emergence of new variants of the virus, as well as the potential for future changes to Australia’s border controls.
Whilst COVID-19 infection can sometimes result in myocarditis, its incidence following COVID-19 vaccination is comparatively extremely rare. Most individuals diagnosed with myocarditis following COVID-19 vaccination have responded well to treatment.
How is pericarditis following COVID-19 vaccination triggered?
The exact mechanism behind cardiac sac inflammation temporally associated with COVID-19 vaccination is currently being investigated. Clinical causes from international surveillance data suggest an immune-mediated or hypersensitivity trigger.
Who is at risk of pericarditis?
Pericarditis from any cause occurs in similar rates amongst males and females. It is also more likely to affect younger adults.
Reported rates of pericarditis occurring following administration of a COVID-19 vaccine vary; however, they are above expected background population rates. Available surveillance data suggests the risks for pericarditis after a mRNA vaccine are higher than non-mRNA vaccines.
International and local data indicate that pericarditis following COVID-19 vaccines is more common in the 18-39 year old age group for both males and females. Pericarditis following third doses or booster doses have also been identified in a small number of individuals. Reported cases have occurred at significantly lower rates than those identified following either dose of a primary course.
Are children more likely to experience pericarditis following COVID-19 vaccination?
Available safety data from local and international sources suggest there is a significantly lower risk of children developing pericarditis following COVID-19 vaccination.
ATAGI recommends an interval of 8 weeks between vaccine doses in a primary course for age-eligible children (≤ 11 years) for maximum efficacy and safety. This extended interval is based on international data suggesting a longer time interval between dose 1 and 2 may reduce the risk of myocarditis – which in principle may also extend to pericarditis. This extended interval also allows more time to observe international vaccine safety data and identify any signals for rare adverse events.
For more information on COVID-19 vaccination in individuals < 18 years please refer to COVID-19 vaccination in children and adolescents.
Pre-existing cardiac conditions and COVID-19 vaccination
Individuals with the following cardiac conditions can safely receive COVID-19 vaccines without the need for additional monitoring or precautions:
- coronary artery disease
- myocardial infarction
- stable heart failure
- arrhythmias
- rheumatic fever
- rheumatic heart disease
- kawasaki disease
- most congenital heart disease
- those with implanted cardiac devices
- congenital heart disease
- cardiac transplant
- cardiomyopathy.
Those with a history of the following conditions can also receive COVID-19 vaccines; however should consult their treating specialist to determine the appropriate timing for vaccination:
- recent (within 3 months) or current inflammatory cardiac conditions (including myocarditis, pericarditis and endocarditis)
- acute rheumatic fever or acute rheumatic heart disease
- acute decompensated heart failure.
Patients with ongoing cardiac inflammation should have vaccination deferred. In some instances, vaccination with Vaxzevria (AstraZeneca) or Nuvaxovid (Novavax) may be considered due to the lower associated risk of pericarditis.
What are the symptoms of pericarditis?
Pericarditis presents similarly to myocarditis, with a range of symptoms including:
- chest pain, pressure or discomfort
- pain with breathing (pleuritic chest pain)
- shortness of breath
- palpitations
- syncope (faint)
- other non-specific symptoms such as fatigue, dizziness, abdominal pain.
How is pericarditis after COVID-19 vaccination diagnosed and investigated?
If there is suspicion of either of these conditions, particularly in the first 2-3 weeks following vaccination, timely medical review is important. Those who appear unwell should be referred to an emergency department for the following investigations:
- blood tests for cardiac biomarkers, such as troponin
- electrocardiogram (ECG)
Other tests should be considered if the patient unwell or the tests above are abnormal:
- chest X-ray (CXR)
- other tests related to investigating differential diagnoses such as inflammatory markers (C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR)).
Consistent findings of pericarditis include a pericardial rub on auscultation, widespread ST-elevation or PR depression on ECG, as well as pericardial effusion on imaging.
Individuals who are investigated for pericarditis following vaccination should avoid high-intensity exercise until symptoms have resolved is recommended. Those experiencing ongoing symptoms should return for review in 1-2 days time. Cardiologist advice is recommended if clinical suspicion is high, regardless of normal investigations.
How is pericarditis after COVID-19 vaccination treated?
Current data shows that most cases of pericarditis following COVID-19 vaccination have mild symptoms and recover well.
In some scenarios, there have been reports of pericarditis causing prolonged and recurrent symptoms but with functionally normal investigations including cardiac imaging. Information on long term sequelae is still being collected.
Treatment of these conditions is managed by a cardiologist and include in-patient supportive therapies, often with anti-inflammatory medications or colchicine. In the rare severe or complicated cases, specific management for arrhythmias, decreased cardiac function or congestive cardiac failure with pharmacological agents such as ACE-inhibitors and beta-blockers or mechanical support may be necessary.
What are the implications for future doses (including third and booster doses)?
For individuals where the cause of inflammation is attributed to COVID-19 vaccination, a report to SAEFVIC is indicated and a referral to a cardiologist and/or specialist immunisation service (eg. VicSIS) is recommended.
Please refer to the below algorithm for recommendations relating to further doses of COVID-19 vaccines in patients who have been diagnosed with pericarditis following vaccination.
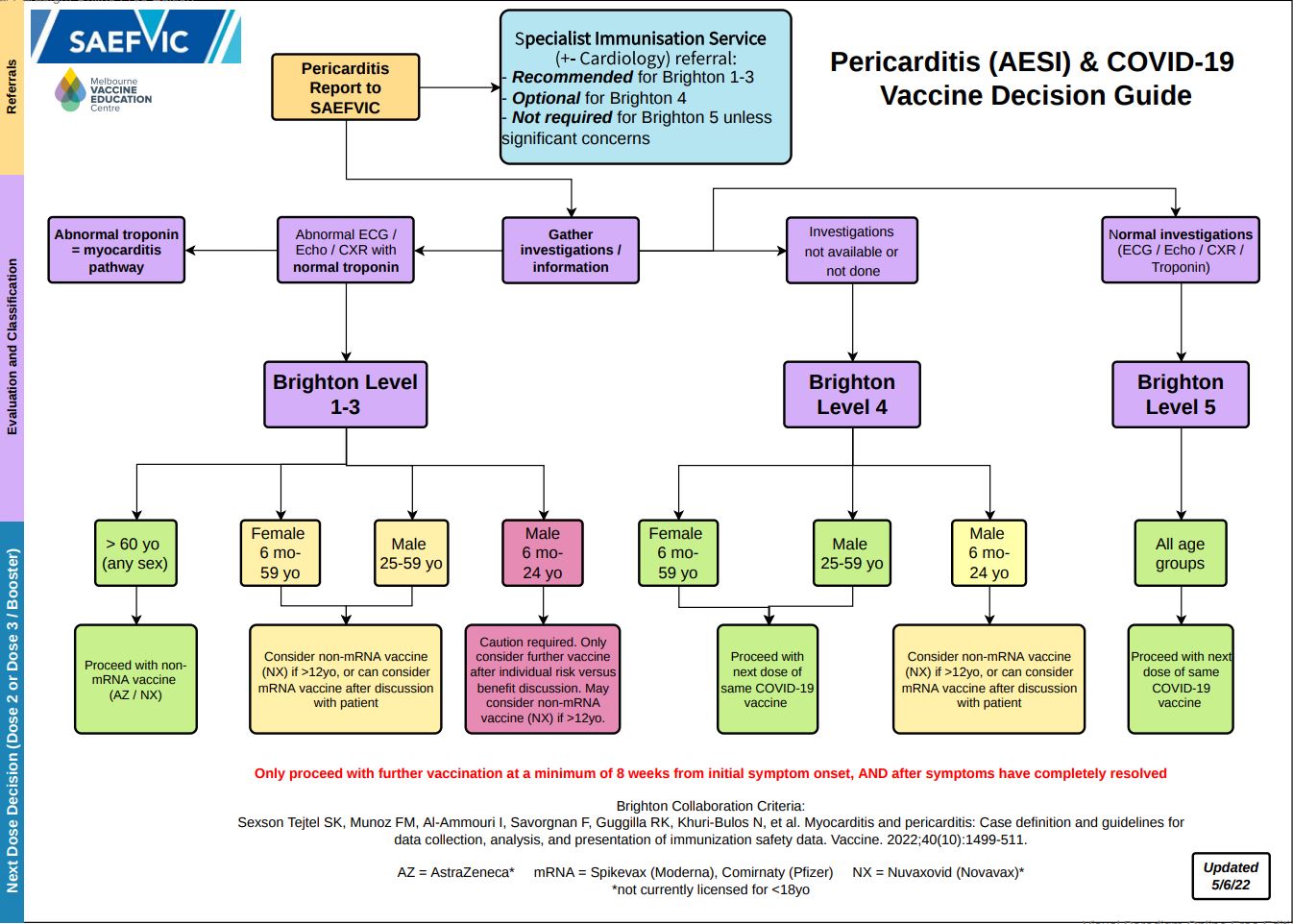 *Brighton Level refers to Brighton Collaboration criteria for classifying pericarditis
*Brighton Level refers to Brighton Collaboration criteria for classifying pericarditis
Access a pdf version of this guide here.In particular, the groups which are at lower risk (green in algorithm), could proceed with further doses of COVID-19 vaccination as per above suggested instructions.
What is the risk/benefit ratio for vaccination for the young adult age group?
It is important to discuss your individual circumstances with a health care provider in order to make an informed decision.
The level of COVID-19 community transmission in Australia can change quickly. Factors to consider include age, potential for exposure to the virus (including in the workplace), high rates of global transmission, the emergence of new variants of the virus, as well as the potential for future changes to Australia’s border controls.
Whilst COVID-19 infection can sometimes result in pericarditis, its incidence following COVID-19 vaccination is comparatively extremely rare. Most individuals diagnosed with pericarditis following COVID-19 vaccination have responded well to treatment.
I am taking certain medications that have myocarditis listed as an uncommon side effect. Am I at greater risk of developing myocarditis/pericarditis after COVID-19 vaccination?
Taking medications that have myocarditis listed as an uncommon side effect (e.g. antipsychotic drugs and biological chemotherapeutic agents) is not a contraindication to COVID-19 vaccination. Individuals taking these medications can be safely vaccinated in the community with no need for additional precautions or monitoring.
Are there impacts of other substances on the development of myocarditis/pericarditis after COVID-19 vaccines?
The use of recreational stimulants (particularly amphetamines) is discouraged especially in the week following vaccination to limit the potential for developing myocarditis/pericarditis.
Should exercise be limited after receiving COVID-19 vaccines to reduce the chance of myocarditis/pericarditis?
Exercise is not thought to increase the risk of developing myocarditis/pericarditis following COVID-19 vaccination. It is therefore not necessary to reduce or avoid exercise in the post-vacccination period.
However, if patients develop myocarditis/pericarditis post vaccination there is a concern that exercise may be pro-arrhythmic (eg. will exacerbate) the condition.
As myocarditis/pericarditis following COVID-19 vaccination is thought to be immune mediated, would those with pre-existing autoimmune diseases be at an increased risk compared to the general public?
Myocarditis/pericarditis following COVID-19 vaccination appears to be idiosyncratic at this stage, with no clear risk factors. Thus, there is no indication of increased risk in those with underlying autoimmune disease.
Resources
Investigation, management and treatment of myocarditis/pericarditis
- HealthPathways Myocarditis and Pericarditis after mRNA COVID-19 Vaccines (login required)
- Paediatric Research in Emergency Departments International Collaborative: Chest pain guideline
Guidelines on Myocarditis/Pericarditis
- COVID-19 vaccination – Guidance on myocarditis ad pericarditis after COVID-19 vaccines
- CDC Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults
- COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) reviews cases of mild myocarditis reported with COVID-19 mRNA vaccines
- Brighton Collaboration: Myocarditis/Pericarditis Case Definition
MVEC resources on COVID-19 vaccines and myocarditis/pericarditis
- The MVEC Conversation: myocarditis/pericarditis following mRNA vaccination
- MVEC COVID-19 vaccine FAQs: mRNA vaccines: myocarditis/pericarditis
Authors: Rachael McGuire (MVEC Education Nurse Coordinator), Francesca Machingaifa (MVEC Education Nurse Coordinator), Daryl Cheng (MVEC Medical Lead) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Daryl Cheng (MVEC Medical Lead) and Julia Smith (Immunisation Fellow, Royal Children’s Hospital)
Date: October 27, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Multi-dose vials
What are they?
Multi-dose vials contain more than one dose of a medicine/vaccine in a single vial. Whilst all vaccines on the National Immunisation Program are single-use preparations, BCG vaccines and COVID-19 vaccines are available in multi-dose vials in Australia. Multi-dose vials are more economical, take less time to manufacture and require less storage space than single-use preparations, however, there is an increased risk of infection control breaches associated with their use.
Infection control principles
There is an increased risk of blood-borne viruses or bacterial contamination with the use of multi-dose vials due to an increased risk of cross contamination. These risks can be mitigated by:
- Maintaining standard principles of infection control and strict aseptic technique when accessing multi-dose vials
- Preparing doses of vaccines from multi-dose vials in a clean, designated medication preparation area
- Cleaning the stopper with an alcohol swab and allowing to dry every time the vial is accessed
- Using a new, sterile syringe and needle each time the vial is accessed. Needles should never be left inside the vial
- Discarding a multi-dose vial if the vaccine’s integrity or sterility is compromised
Storage and usage
- Follow the manufacturer’s recommendations for refrigeration, storage, usage timeframes and expiry dates. Protect from sunlight and freezing where required.
- Always label a multi-dose vial with the date and time of first access or reconstitution
- The expiry date is the date after which an unused multi-dose vial should be discarded
- The use by date is the date after which a multi-dose vial that has been accessed should no longer be used. A use by date supercedes the expiry date
- Check reconstituted vaccines for signs of deterioration, such as a change in colour or clarity. If there are signs of deterioration, refer to the vaccine product information. Do not use the vaccine
Multi-dose vials that require reconstitution
- Only the recommended diluent should be used to reconstitute a multi-dose vial
- Introduce the diluent down the side of the vial to avoid foaming or potentially denaturing the vaccine. Mix gently with a careful swirling motion. Do not shake
- Give reconstituted vaccines as soon as practicable after reconstituting. This is because reconstituted vaccines may deteriorate rapidly
- Once accessed, label the multi-dose vial with the date and time of reconstitution
Pre-filling syringes
Pre-preparing syringes with vaccines is not recommended for several reasons:
- The uncertainty of vaccine stability
- The risk of contamination
- Increased risk of potential errors in administration
- Potential vaccine wastage
If you are in a setting where pre-preparing multiple doses is required, then only draw up the number of doses necessary to keep the immunisation session running efficiently. These doses must be labelled with the date and time the vial was accessed and should be used as soon as possible, ensuring that the cold chain is maintained.
Principles of administration
- Attach a new, sterile, disposable injecting needle of appropriate size and length to administer the vaccine
- Be careful not to prime the needle with any of the vaccine as this can increase the risk of injection site reactions
- Administer the vaccine as soon as practicable after drawing it up
- Discard multi-dose vials at the end of an Immunisation session/6 hours after accessing (whichever is sooner) or according to manufacturer’s guidelines
- Refer to the product information to determine the specified timeframe the vaccine must be used by once the vial has been accessed
Resources
- MVEC: Use of Multi-dose Vials eLearning package
- ATAGI guidance on the use of multi-dose vials for COVID-19 vaccination
- ACIPC: Aseptic Technique Clinician Cheat Sheet
- Canadian Immunization Guide: Vaccine administration practices
- Center for Disease Control Injection Safety: Questions about multi-dose vials
- Medical Journal of Australia: Vaccination, consent and multi-dose vials
- WHO policy statement: Multi-dose Vial Policy (MDVP)
Authors: Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: March 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
MTHFR gene
Background
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that helps the body to break down folate. The MTHFR enzyme is made by the MTHFR gene. Harmless changes in the MTHFR gene, polymorphisms, are very common. Importantly, MTHFR polymorphisms do not cause any significant health problems. MTHFR gene variants or mutations are different to gene polymorphisms and are very rare. For more information about the MTHFR gene, refer to the resource below [Resources: VCGS MTHFR].
MTHFR gene polymorphisms and vaccines
People who have MTHFR gene polymorphisms can safely receive vaccines. There is no increased risk of adverse events following immunisation (AEFI).
MTHFR gene polymorphism testing
There is no clinical indication for MTHFR polymorphism testing before vaccination.
Authors: Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Margie Danchin (Senior Research Fellow, Murdoch Children’s Research Institute)
Reviewed by: Katie Butler (MVEC Education Nurse Coordinator) and Sally Gordon (MVEC Senior Research Fellow)
Date: October 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Maternal vaccination during pregnancy
Immunisation assessment is an extremely important aspect of healthcare during pregnancy. When planning a pregnancy, talk to your health care provider about any vaccines you might need beforehand. Live-attenuated vaccines should be given at least a month before conception.
Recommended vaccines
Influenza, pertussis and COVID-19 vaccines are the only vaccines routinely recommended for pregnant women. They are provided for free via the National Immunisation Program (NIP).
Some other vaccines can be administered in special circumstances but are not routinely recommended. Refer to the Australian Immunisation Handbook for further information.
Influenza
Influenza vaccination is safe and strongly recommended for pregnant women to avoid complications from influenza disease. It can be administered at any stage of pregnancy and not only aims to protect the expectant mother from disease, but also to provide passive protection to the infant.
Pregnant women are at greater risk of morbidity and mortality from influenza disease than non-pregnant women. They are more than twice as likely to be hospitalised with influenza disease as other people with influenza.
Babies less than 6-months of age are at greatest risk of disease and death from influenza and maternal vaccination will provide protection to babies for the first few months of life until they can be immunised against influenza from 6-months of age.
Pertussis
Pertussis (whooping cough) immunisation during pregnancy is a safe and effective way to protect the mother and prevent disease of the newborn. It is recommended that a single dose of the vaccine be administered between 20 and 32 weeks of pregnancy, in every pregnancy, including pregnancies that are closely spaced.
Maternal antibodies against pertussis provide protection for babies until they have at least received 2 doses of their own pertussis containing vaccine (given at 6-weeks and 4-months of age). Babies less than 6-months of age are at greatest risk of severe disease and death from pertussis.
COVID-19 vaccines
Due to an increased risk of severe outcomes for pregnant women and their unborn babies it is recommended that pregnant women are routinely offered COVID-19 vaccines. Vaccines can be given at any stage of pregnancy.
Surveillance of international data on administration of mRNA COVID-19 vaccines (Comirnaty (Pfizer) or Spikevax (Moderna)) to pregnant women has shown no significant safety concerns for either the mother or the baby. Evidence demonstrates antibodies can pass into breastmilk and cord blood which may provide protection to infants via passive immunity.
Nuvaxovid (Novavax) may be administered to pregnant and breastfeeding women however there is no immunogenicity or safety data on it’s use in this patient group.
Pregnant women have been shown to have an increased risk of needing admission to the intensive care unit and requiring mechanical ventilation if they contract COVID-19 compared with non-pregnant women of the same age.
Women who are planning pregnancy or who are breastfeeding can safely receive a COVID-19 vaccine. You do not need to stop breastfeeding before or after vaccination.
For more information refer to the following:
- Joint statement between RANZCOG and ATAGI about COVID-19 vaccination for pregnant women
- COVID-19 vaccination- ATAGI clinical guidance on COVID-19 vaccine in Australia in 2021
- COVID-19 vaccination- COVID-19 vaccination decision guide for women who are pregnant, breastfeeding or planning pregnancy
- AJOG: COVID-19 vaccine response in pregnant and lactating women: a cohort study
Contraindicated vaccines
All live-attenuated vaccines are contraindicated during pregnancy due to the potential risk to the unborn baby [see Table 1 below]. In most circumstances the risk is hypothetical however, there is insufficient evidence to support vaccination in this patient group. The limited safety data from inadvertent administration of live-attenuated viral vaccines such as the MMR and Varicella vaccines is reassuring.
Table 1: Live-attenuated vaccines contraindicated in pregnancy
| Disease | Brand name |
|---|---|
| Rotavirus | Rotarix®, Rotateq® |
| MMR (measles-mumps-rubella) | Priorix®, MMR II® |
| MMRV (measles-mumps-rubella-varicella) | Priorix-tetra®, ProQuad® |
| Varicella (chickenpox) | Varilrix®, Varivax® |
| Zoster (shingles) | Zostavax® |
| Tuberculosis | BCG (varying brands) |
| Yellow fever | Stamaril® |
| Typhoid^ | Vivotif® |
| Japanese encephalitis | Imojev® |
^Oral vaccine
Resources
- COVID-19 vaccination- ATAGI clinical guidance on COVID-19 vaccine in Australia in 2021.
- COVID-19 vaccination- COVID-19 vaccination decision guide for women who are pregnant,breastfeeding or planning pregnancy
- Raising Children Network: COVID-19 vaccination: preganancy and breastfeeding
- Gray, K.J et al COVID-19 vaccine response in pregnant and lactating women: a cohort study American Journal of Obstetrics and Gynecology
- ABC News report: ‘Antivaxxer’ tells of ‘nightmare’ after passing whooping cough onto baby daughter
- Amirthalingam, G. et al Effectiveness of maternal pertussis vaccination in England: an observational study The Lancet October 2014 384(9953) 1521-1528
- Steedman, M. et al Strategies to boost maternal immunization to achieve further gains in improved maternal and newborn health Health Affairs 2016 (35)309-316
- Australian Immunisation Handbook: Vaccination for women who are planning pregnancy, pregnant or breastfeeding
- Australian Government Department of Health: Influenza and pertussis vaccination in pregnancy
- Year Round Influenza Vaccination FAQ
Monash Health immunisation resources
- 2019 Patient Information sheet Influenza vaccine during pregnancy
- 2019 Patient information sheet Boostrix during pregnancy
- Patient information sheet Rubella Vaccine
MVEC resources
- MVEC: Vaccines in Pregnancy eLearning package
- MVEC: Pertussis
- MVEC: Influenza vaccine recommendations
- MVEC: Breastfeeding and immunisations
- MVEC: COVID-19 vaccine FAQs: women’s health
Authors: Michelle Giles (Infectious Diseases Consultant, Monash Health) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: November 30, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Measles
What is it?
Measles, also known as rubeola, is a highly contagious viral illness. It is caused by the measles virus which belongs to the Morbillivirus family.
What to look for
Infection usually begins with 3-4 days of fever, malaise, cough, coryza (runny nose) and conjunctivitis. Small white spots, known as Koplik spots, are also present on the buccal mucosa (mucosal surface of the cheeks) of an infected person.
3-4 days later a maculopapular rash (red with a combination of flat and raised areas) then develops, often beginning on the face before becoming more generalised and can last up to 7 days. Very rarely does a measles infection occur in the absence of a rash.
Complications of a measles infection include pneumonia and otitis media (ear infection). Approximately 1 in 1000 people will develop encephalitis (brain inflammation) which carries a mortality rate of 10-15%. Sub-acute sclerosing panencephalitis (SSPE) is a rare progressive neurological disorder that can develop 2-10 years after an initial measles infection. It is characterised by encephalitis and demyelination (loss of the protective covering of the nerve fibres) causing behaviour changes, seizures and muscle rigidity. SSPE is fatal in all cases.
Measles infections during pregnancy can result in miscarriage and prematurity.
How is it transmitted?
Measles can be spread by an infected person coughing or sneezing respiratory droplets or through the direct contact with infected nasal or throat secretions. An environment can remain infectious for up to 2 hours after an infectious person has been present.
The incubation period is 7 to 18 days (more commonly 10 days), with a person able to transmit disease for up to 5 days prior to the onset of the rash, and as long as 4 days after the rash develops.
Humans are the only reservoirs for the measles virus.
Epidemiology
Prior to the introduction of vaccination in 1963, measles infections contributed to 2.6 million deaths globally each year. Despite extensive vaccination campaigns worldwide, measles infection still carries a significant burden in some countries contributing to 142 000 deaths globally in 2018.
Within Australia, however, high rates of vaccine coverage led to the WHO declaring Australia “measles-free” in 2014. Measles infections are still seen in non-immune individuals travelling internationally, placing unimmunised Australians at risk of infection.
Prevention
Live-attenuated measles-containing vaccines are highly effective in protecting against disease. A 2-dose course of vaccination is routinely provided on the National Immunisation Program (NIP) for children as combination vaccines at:
- 12 months of age – Priorix®/M-M-R®II (measles- mumps- rubella (MMR))
- 18 months of age – Priorix-Tetra®/ProQuad® (measles-mumps-rubella-varicella (MMRV))
In addition, babies aged between 6 months and 11 months travelling overseas, non-immune women planning pregnancy or non-immune women after delivery, and any person born since 1966 who does not have evidence of 2 doses of vaccination/is seronegative is eligible to receive funded measles-containing vaccines.
Due to high rates of circulating measles virus in the years preceding 1966 and the life-long immunity invoked by natural infection, those born during this time are considered already immune and generally do not require vaccination.
Contraindications
Live-attenuated vaccines such as measles-containing vaccines are contraindicated in individuals with immune compromise due to the risks of adverse events and the chance of developing vaccine-related disease.
Additionally, pregnant women should not receive measles-containing vaccines due to the potential risks to the unborn baby. Instead, vaccination at least 4 weeks prior to pregnancy or vaccination in the postnatal period is recommended.
Precautions
Specific intervals between the administration of immunoglobulins or other blood products and administration of measles-containing vaccines are recommended. This is due to the potential for any circulating donated antibodies affecting the immune response to vaccination.
Side effects
7-10 days following MMR vaccination, individuals may experience fever, malaise, and a non-infectious rash lasting 2-3 days.
MMRV vaccines are not recommended as a first dose of a measles-containing vaccine in children < 4 years of age due to the increased risk of fever and febrile seizures.
Post-exposure prophylaxis
If a non-immune individual is exposed to measles, immunisation with MMR or MMRV is recommended to occur within 72 hours of exposure to reduce the likelihood of infection (provided immunisation is not a contraindication).
Infants ≤ 5 months of age born to non-immune mothers or mothers with < 2 documented doses of measles vaccination, individuals of any age with immune compromise, and pregnant women, may be recommended to receive Normal Human Immunoglobulin (NHIG) if exposed to disease [refer to resources].
Resources
- Australian Academy of Science: measles- everything you need to know
- Australian Immunisation Handbook: Table: Post-exposure prophylaxis needed within 72 hours of 1st exposure for people exposed to measles
- Australian Immunisation Handbook: Table: Recommended intervals between immunoglobulins or blood products, and measles-mumps-rubella, measles-mumps-rubella-varicella or varicella vaccination
- World Health Organization: measles
- MVEC: MMR vaccine and autism
- RCH Clinical Practice Guideline: illness in the returned traveller
- Better Health Channel: measles
Author: Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: December 8, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
MMR vaccine and autism
Some families have concerns around the measles-mumps-rubella (MMR) vaccine and the diagnosis of autism. At MVEC we encourage parents to find the best available evidence to help them make a decision around vaccinating their child. The MMR live-attenuated vaccine is recommended on the National Immunisation Program at 12-months of age and combined with the varicella vaccine (MMRV) at 18-months of age.
A great resource for addressing MMR concerns is a decision aid developed by the National Centre for Immunisation Research and Surveillance (NCIRS) in Sydney. It is targeted at:
- Parents or caregivers of a child approaching their due date for MMR vaccination.
- Anyone who would like more information about MMR vaccination
Note: this guide provides general information only, and is not intended as a substitute for consultations with qualified health professionals. For specific queries talk with your local doctor or immunisation provider, or contact us at [email protected] or Telephone: 1300 882 924
Resource
- NCIRS: MMRV decision aid
- SKAI: What about autism?
- Vaccines and Autism: Children’s Hospital of Philadelphia
Authors: Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Date: July 2020
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.