疫苗接种
大多数可在澳大利亚使用的疫苗都是通过肌内或皮下途径给药,其中一些是皮内或口服给药。通过推荐的途径接种疫苗并使用正确的技术对于确保最佳的免疫反应、最大限度地减少副作用和降低患者受伤的风险至关重要。
给药途径
肌肉注射
肌肉注射疫苗以 90° 角进入组织的肌肉层。肌肉含有大量血管,导致血液供应增加,从而使疫苗更容易分散。此外,肌肉含有树突状(抗原呈递)免疫细胞,可启动持久的免疫反应。除了注射部位发红和疼痛外,肌内注射疫苗的副作用往往较小,这些副作用通常较轻微且持续时间较短。
皮下
皮下疫苗以 45° 角进入皮下或皮下脂肪层。设计用于皮下注射的疫苗以更慢且更恒定的速度被吸收,因为皮下组织的血液供应少于肌肉。
皮内
皮内疫苗以 5-15° 角注射到表皮和真皮之间的皮肤外层。表皮和真皮层包含许多不同的细胞,包括抗原呈递细胞 (APC),这些细胞被认为在介导对特定疫苗的有效和保护性免疫反应中发挥重要作用。 3 种主要的 APC 是巨噬细胞、树突状细胞和 B 细胞。 APC 将特定抗原呈递给免疫系统中的特定细胞,这些细胞负责引发免疫介导的反应,进而产生记忆细胞和抗体。
皮内接种疫苗是一种独特的疫苗接种方法,需要经过专门培训才能确保疫苗的安全性和有效性。
口服
口服疫苗可激活胃肠道粘膜中的免疫细胞,这有利于预防影响肠道的疾病。
根据疫苗的不同,口服疫苗可以采用片剂或液体制剂。建议口服的液体疫苗不应注射。片剂形式的口服疫苗不应咀嚼或弄碎。
请注意,如果接受口服疫苗的婴儿吐出少量疫苗,这仍被视为有效剂量,无需重复接种。如果他们在接种后几分钟内吐出大部分剂量的疫苗,则应在同一次就诊时重复接种。
注射疫苗
肌内和皮下注射
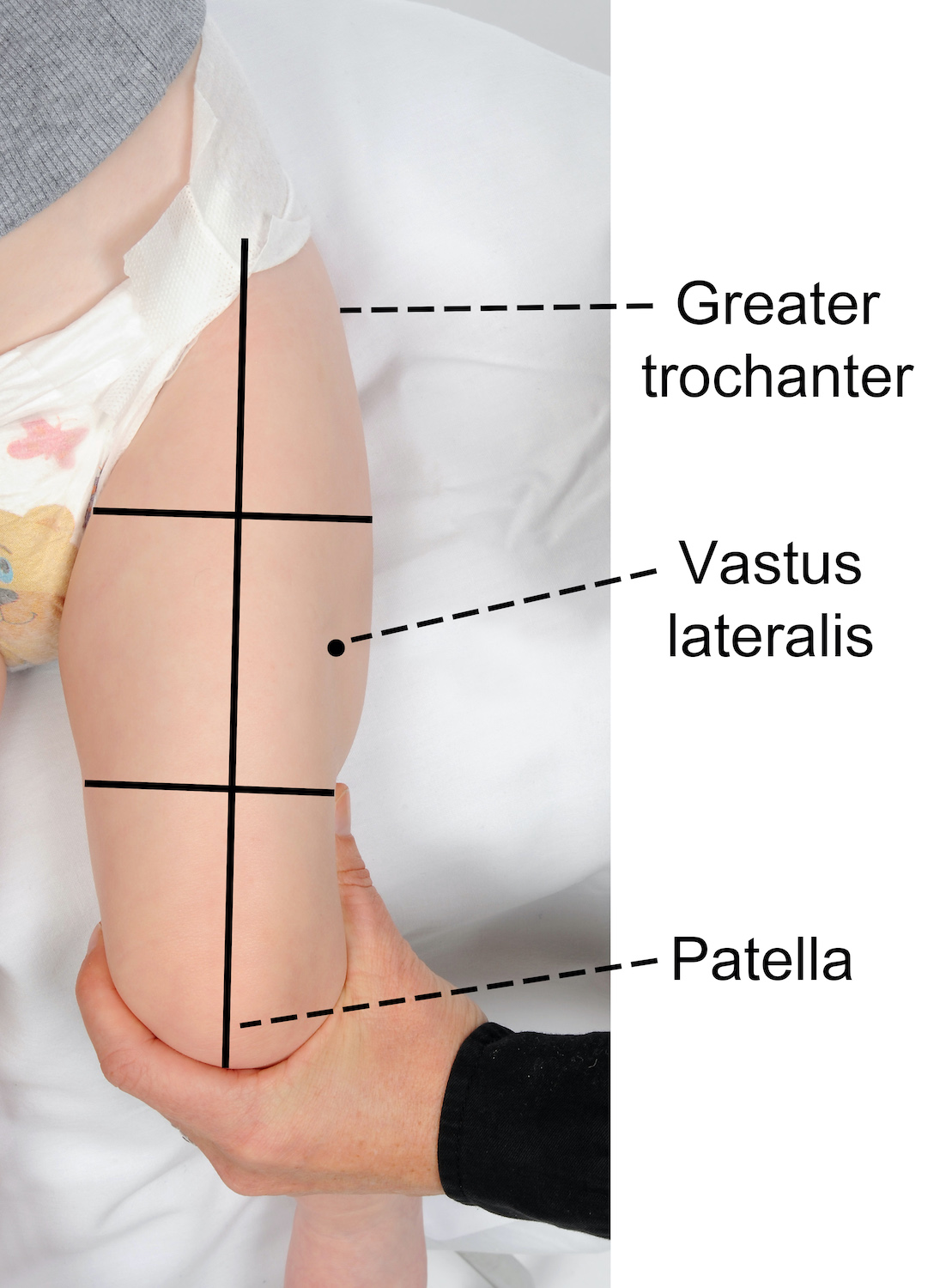
12 个月以下婴儿的推荐注射部位
推荐的注射部位是股外侧肌的中间三分之一(大腿前外侧)。
要找到正确的注射解剖部位:
- 确保婴儿的腿完全暴露
- 定位上下解剖学标志-股骨大转子和髌骨
- 在大腿前部下方的 2 个地标之间画一条假想线
- 然后想象大腿被分成三等份
- 正确的注射部位位于中间三分之一和假想线的外侧(见下图)。
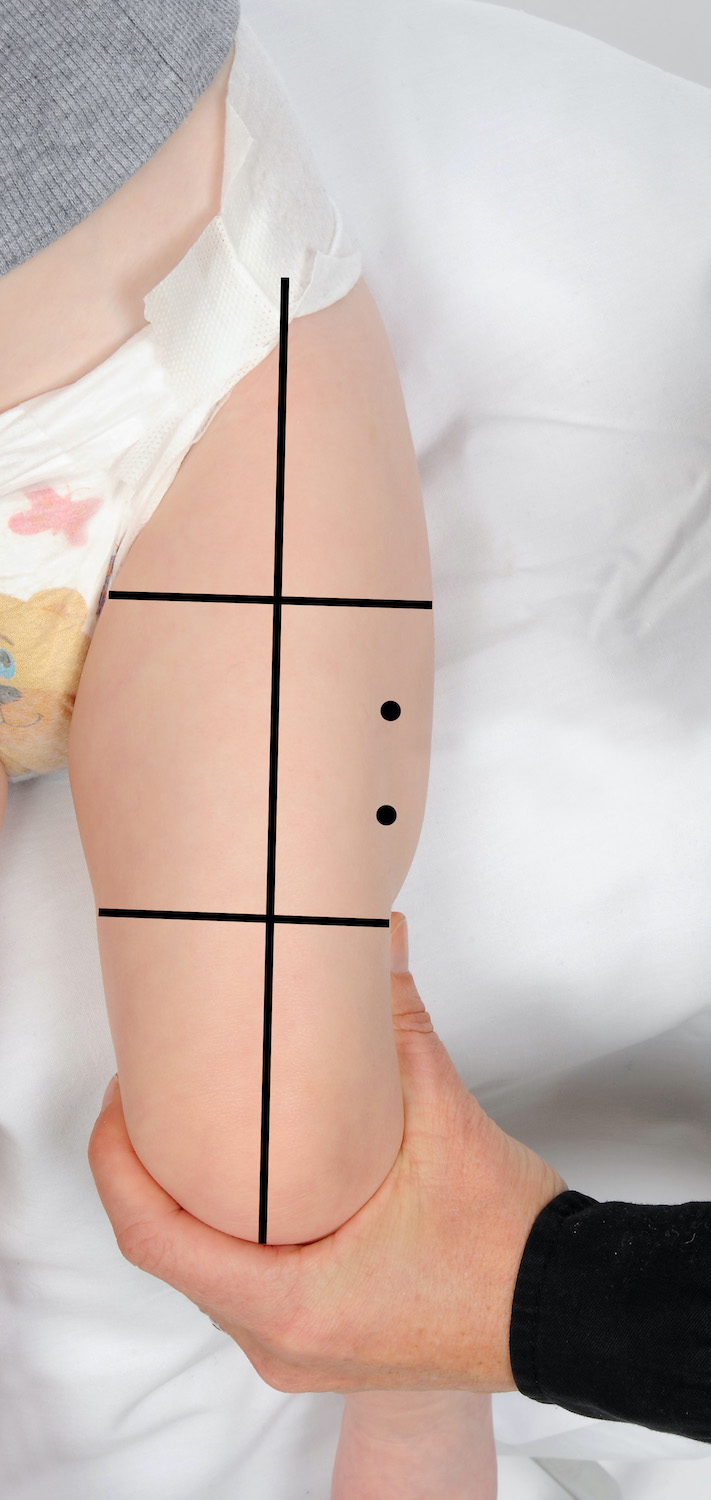
在哪里 只有两种疫苗 建议每条大腿注射一剂疫苗。如果 两种以上疫苗 建议在一次就诊时,可以在每条大腿上注射两种疫苗,确保它们之间的距离为 2.5 厘米(参见下图)。当在同一条大腿中注射多种疫苗时,重要的是要考虑每种疫苗的反应原性。那些被认为更具反应原性的部位应尽可能位于不同的大腿。例如,脑膜炎球菌 B (Bexsero®) 通常与局部部位反应增加有关。
在某些无法使用大腿的情况下(例如先天性肢体畸形、活动性湿疹或髋部支架放置),可以使用三角肌(请参阅≥12个月儿童的推荐注射部位以获取指导)。
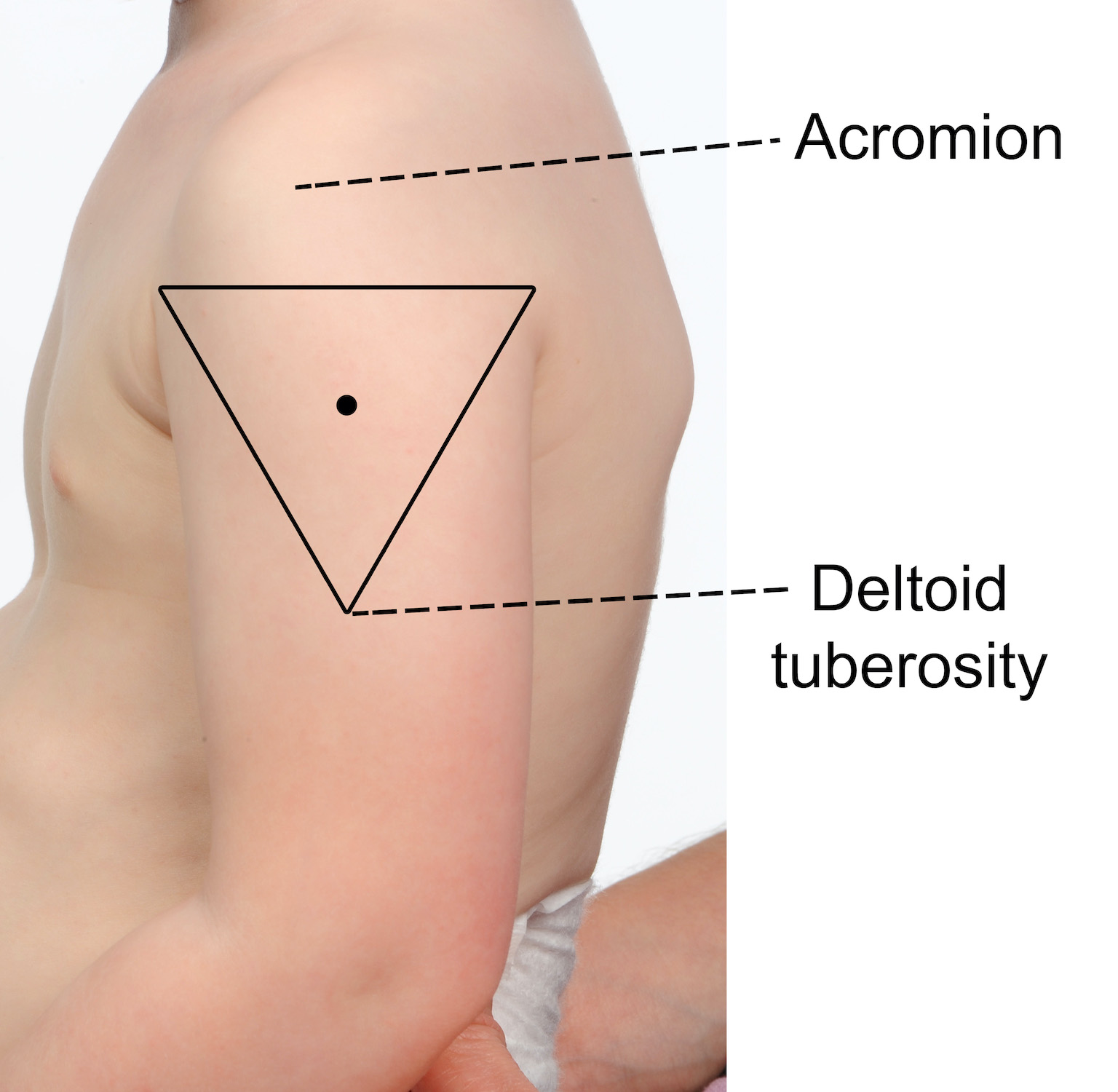
≥ 12 月龄儿童、青少年和成人的推荐注射部位
推荐的注射部位是三角肌(上臂)。
要找到正确的注射解剖部位:
- 从肩膀顶部到肘部完全暴露手臂;如果需要,脱下衬衫或衣服
- 定位上部和下部解剖学标志 - 肩峰(肩尖)和三角肌的肌肉插入(三角肌结节)
- 使用已识别的地标在肩尖下方绘制一个假想的倒三角形(见下图)
- 注射部位在肩峰与三角肌结节之间,三角肌中部(三角)。
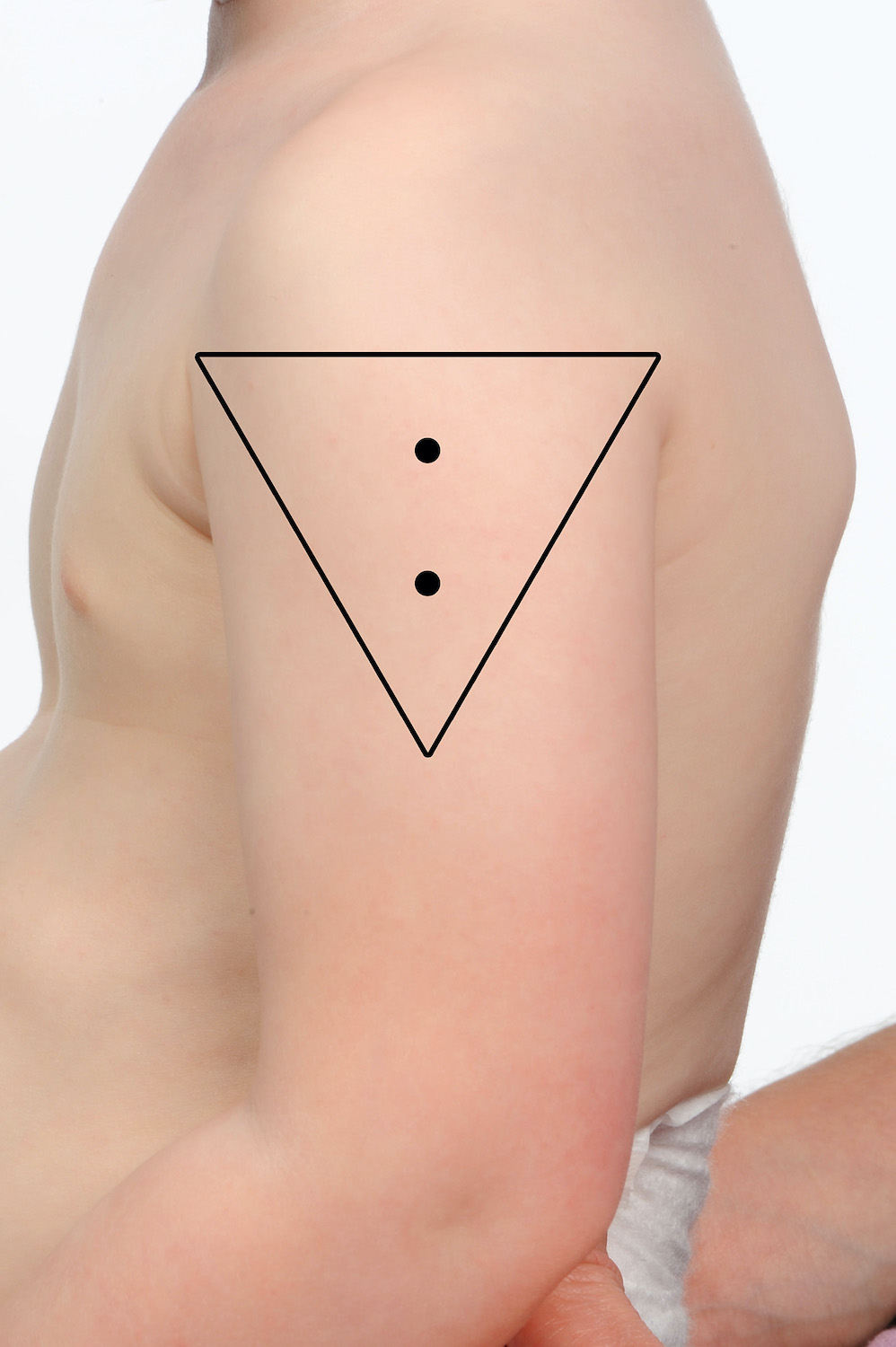
两种或多种疫苗 可以将其放入同一个三角肌中,确保它们分开 2.5 厘米(参见下图)。当在同一三角肌中注射多种疫苗时,重要的是要考虑每种疫苗的反应原性。那些被认为更具反应原性的人应该尽可能位于不同的三角肌中。例如,脑膜炎球菌 B (Bexsero®) 和肺炎球菌多糖 (Pneumovax® 23) 通常与局部部位反应增加有关。
该年龄组也可能需要利用大腿前外侧(例如先天性肢体畸形、腋窝间隙病史、活动性湿疹或可用间距不足)。在这些情况下,优先通过该部位注射反应原性最低的疫苗(请参阅 ≤ 12 个月儿童的推荐注射部位以获取指导)。
皮内注射
任何年龄的皮内注射推荐部位
推荐的皮内给药部位是前臂中部的掌侧(内)表面或三角肌插入肱骨的三角肌区域上方。
左臂三角肌区域特别推荐用于任何年龄组的 BCG 给药,因为在此部位给药可最大限度地降低瘢痕疙瘩形成的风险。
哪些疫苗可以皮内注射?
通过 ID 途径接种的疫苗示例包括 BCG 和 乙型肝炎 (对于无反应者)。曼图和 问发烧 测试也在皮内进行。
乙型肝炎疫苗通常通过肌内注射途径接种,但有一小部分人对乙型肝炎疫苗的肌内注射过程没有产生保护性免疫反应。如果他们的提供者认为他们是无反应者,则皮内途径被认为是替代方案 [请参阅 MVEC:乙型肝炎 了解更多信息]。
WordPress Tables Plugin疫苗 剂量 推荐网站 卡介苗(结核) < 12 个月 0.05ml
≥ 12 个月 0.1ml左上臂在三角肌插入肱骨的区域上方§ Engerix®-B 成人(乙型肝炎)¥ 每剂 0.25 毫升(20 微克/1.0 毫升) 左上臂超过三角肌区域 Jynneos® (MPX) 0.1毫升 左上臂在三角肌区或前臂中部的掌侧 结核菌素皮肤试验 (TST/Mantoux) 0.1毫升 前臂中段的掌面 Q-Vax皮肤测试(Q发烧测试) 0.1ml 稀释的 Q-Vax 皮肤测试 前臂中段的掌面 §这是将瘢痕疙瘩形成风险降至最低的推荐部位
¥参考 MVEC:乙型肝炎 有关无反应者管理的更多信息皮内接种技术
- 使用带有短斜面的短(10 毫米)26-27 号针头和 1 毫升胰岛素注射器
- 接种 BCG 疫苗时佩戴护目镜,因为溅入眼睛会导致溃疡
- 确定正确的注射部位(见上表 1)
- 拉伸手指和拇指之间的皮肤
- 将斜角面朝上插入真皮,插入距离约为 1.5 厘米。 2 毫米。斜角应该可以透过表皮看到
注射时您应该感觉到阻力,如果没有,针头可能在皮下组织中。如果注射不是皮内注射,则拔出针头并在新部位重复注射。如果正确给药,皮内注射应该会产生变白的水泡。
准备注射部位
接种疫苗前,注射部位的皮肤应明显清洁。注射前不需要擦拭干净的皮肤。如果皮肤明显变脏,请用酒精清洗/一次性酒精棉签清洁该部位,并在注射前让该部位完全干燥。如果推荐的部位出现活动性/感染性湿疹,请考虑更换部位以最大限度地降低发生湿疹的风险 注射部位脓肿.如果没有合适的替代部位,请考虑使用酒精清洗液/一次性酒精棉签清洁该部位,并在注射前让该部位完全干燥。
用于接种疫苗的推荐针头尺寸、长度和角度
| 患者的年龄或体型 | 针型 | 进针角度 |
| 用于肌内注射的婴儿、儿童或成人 | 22 – 25 号,长度 25 毫米 | |
| 用于肌内注射的早产儿或非常小的婴儿 | 23 – 25 号,长度 16 毫米 | |
| 肌肉注射的非常大/肥胖患者 | 22 – 25 号,长度 38 毫米 | |
| 所有患者皮下注射 | 25 – 27 号,长度 16 毫米 | |
| 所有患者皮内注射 | 26 – 27 号,长度 10 毫米 | 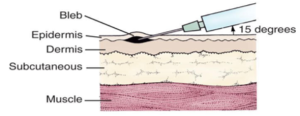 |
* 表格改编自 澳大利亚免疫手册
疫苗接种定位
婴幼儿
重要的是,儿童在接种疫苗期间保持静止,以确保疫苗可以注射到正确的解剖部位,并降低任何意外伤害(例如针刺伤)的风险。在决定孩子是否可以独立坐着或需要支持时,让孩子和父母/监护人都参与讨论很重要。为儿童定位接种疫苗时,重要的是要确保他们感到舒适并且接受疫苗的肢体的活动关节稳定。免疫提供者必须能够充分看到解剖标志和正确的注射部位(三角肌或大腿前外侧)。
可以将婴儿抱在怀里或放在父母膝上,面向提供者,父母/监护人牢牢握住他们的手臂,让他们的大腿完全暴露。年幼的孩子可以抱在怀里或跨坐在他们的上臂完全暴露的位置,如下面的剪辑所示。
对于接受口服疫苗的婴儿,建议将他们放置在可以通过将疫苗管轻轻挤压到他们的内颊来接种疫苗的位置,例如喂食位置。
年龄较大的儿童、青少年和成人
建议年龄较大的儿童、青少年和成人坐在直背椅上,双脚着地进行疫苗接种。提供者应鼓励疫苗接种者放松前臂并将手放在大腿上部,保持手臂在肘部弯曲以帮助放松三角肌。
容易发生血管迷走神经反应(昏厥)的疫苗接种者应躺着接种疫苗,以防止跌倒造成意外伤害。
作者: Mel Addison(默多克儿童研究所 SAEFVIC 研究护士)和 Rachael McGuire(默多克儿童研究所 SAEFVIC 研究护士)
审核人: Francesca Machingaifa(MVEC 教育护士协调员)和 Rachael McGuire(MVEC 教育护士协调员)
日期: 7 月 6, 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
疫苗相关增强性疾病 (VAED)
背景
Vaccine-associated enhanced disease (VAED) is a rare phenomenon where a person who has been vaccinated experiences A (usually) more severe clinical presentation of an infection than would normally be seen in an unvaccinated person. The mechanism秒 for how VAED occurs 是 complex and not clearly understood. Mechanisms may include 这 behav我our 的 Antibodies generated from vaccination 和 abnormal T-cell responses. With the exception of dengue vaccination, where use is restricted to minimi秒e VAED risk (see below), vaccines that have been associated with VAED are no longer in use.
诊断
It can be difficult to distinguish between breakthrough disease (when disease occurs in a vaccinated individual because of inadequate vaccine protection) and VAED. To identify a case of VAED, it is necessary to have a clear understanding of the clinical presentation and usual course of the natural disease. VAED is identified by comparison to the natural disease; the clinical presentation of VAED is atypical, modified or more severe. Factors to be considered when assessing a possible case of VAED include:
- expected background rates of disease
- age
- sex
- time of symptom onset after vaccination
- duration of disease
- clinical course and progression of disease
- any co-morbidities.
Various laboratory and clinical diagnostic parameters can be used to help assess the possibility of VAED, including detailed review of all cases of possible vaccine failure.
Associations
Vaccines that have been associated with the development of VAED include an inactivated respiratory syncytial virus (RSV) vaccine, an inactivated measles vaccine and a vaccine for dengue fever. (Note that the RSV and measles vaccines associated with VAED are not in use in Australia.)
VAED was observed in the 1960s during clinical trials for an inactivated whole-virus vaccine against RSV. In these trials, some children who had received the vaccine and then were later diagnosed with RSV infection developed more serious disease. As a result, this vaccine was never approved for general use.
People who received an inactivated measles vaccines in the 1960s were found to be more likely to develop an atypical form of measles disease if they were infected. This vaccine is no longer available, and the current measles-containing vaccines are not associated with the same adverse effects.
Dengvaxia, a live-attenuated recombinant dengue vaccine that is currently the only licensed dengue vaccine globally, has also been associated with VAED. If given to someone who has never had dengue infection, Dengvaxia can increase the risk of serious disease if the person is infected with dengue after vaccination. For this reason, Dengvaxia is only recommended in specific groups of people who have already had dengue infection, for prevention of subsequent, more serious secondary infection, within closely defined criteria.
Given the rare possibility of VAED, careful surveillance for potential VAED is an important part of both development of new vaccines, and post-licensure vaccine safety surveillance.
对未来剂量的影响
All vaccines currently available through the 国家免疫计划 (NIP) are not linked to VAED.
COVID-19 vaccines are not linked to VAED.
Dengvaxia is registered by the Therapeutics Goods Administration (TGA), but is not currently marketed in Australia.
资源
- Brighton Collaboration Case Definition of the term “Vaccine Associated Enhanced Disease”
- Gartlan C, Tipton T, Salguero FJ, Sattentau Q, Gorringe A, Carroll MW. Vaccine-associated enhanced disease and pathogenic human coronaviruses. Front Immunol. 2022;13. doi:10.3389/fimmu.2022.882972
- CHOP: Antibody-dependent enhancement (ADE) and vaccines
- Munoz FM, Cramer JP, Dekker CL, et al. Vaccine-associated enhanced disease: Case definition and guidelines for data collection, analysis and presentation of immunization safety data. 疫苗. 2021;29(22):3053–3066. doi:10.1016/j.vaccine.2021.01.055
- ATAGI: Clinical advice of the use of Dengvaxia for Australians
- Bigay J, Le Grand R, Martinon F, Maisonnasse P. Vaccine-associated enhanced disease in humans and animal models: Lessons and challenges for vaccine development. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.932408
作者: Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute), Adele Harris (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Georgina Lewis (SAEFVIC Clinical Manager, Murdoch Children’s Research Institute), Julia Smith (RCH Immunisation Fellow) and Francesca Machingaifa (MVEC Education Nurse Coordinator)
审核人: 阿黛尔哈里斯 (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Ingrid Laemmle-Ruff (免疫接种 Consultant SAEFVIC, Murdoch Children’s Research Institute) 和 RachAel McGuire (MVEC 教育护士协调员, Murdoch Children’s Research Institute)
日期: April 2024
本节内的材料将随着新信息的出现而进行更新。MVEC职员定期审阅材料的准确性.
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
疫苗平台
背景
Vaccines work by activating an individual’s immune system to form antibodies and memory cells in response to a pathogen (disease-causing organism), without the individual having to be infected with that pathogen. This means that if or when that pathogen is encountered in the future, the immune system will be able to respond effectively to fight infection, either minimising the symptoms experienced or preventing disease altogether.
Vaccines can be categorised into types based on the technology that they use to initiate an immune response. This technology is referred to as a ‘vaccine platform’.
灭活疫苗
Inactivated vaccines are created by using killed or deactivated pathogens or parts of pathogens that are unable to replicate and cause symptoms of disease. This approach to vaccine manufacturing has been used for decades to produce vaccines such as those for hepatitis A and B, polio and the polysaccharide pneumoccal (Pneumovax 23) vaccine. Nuvaxovid (Novavax) is an example of an inactivated protein-based 新冠肺炎 vaccine which utilises only part of the virus (the spike protein).
A benefit of these vaccines is that they are safe to administer to most people including 孕 或者 breastfeeding women or those with 免疫妥协. However, the immune response induced by this mechanism alone may not be as strong or long-lasting compared with that from vaccines using other platforms. To overcome this challenge, booster doses or an adjuvant (ingredient added to vaccines during their manufacture to promote a stronger immune response and better disease protection) may be required.
减毒活疫苗
Live-attenuated vaccines contain a whole pathogen that has been weakened (attenuated) in a laboratory making it less capable of replicating and causing disease. Examples of live-attenuated vaccines include the 麻疹, mumps, rubella, 水痘(水痘) 和 rotavirus vaccines.
Live-attenuated vaccines typically induce a strong immune response and provide long-lasting immunity meaning fewer doses are required. The main disadvantage of using this platform is that these vaccines are not recommended for use in people who are immunocompromised due to the theoretical risk of causing vaccine-associated disease or to pregnant women due to the potential harm to the unborn baby. In addition, they take longer and are more difficult to mass produce because the pathogen needs to be grown under enhanced biosafety protocols in specialised laboratories.
Genetic vaccines
Genetic vaccines use one or more of a pathogen’s genes (DNA or mRNA) to invoke an immune response. The COVID-19 vaccines Corminaty (Pfizer) and Spikevax (Moderna) are examples of mRNA vaccines and they contain the genetic code specific to the spike protein portion of the virus.
Genetic vaccines can be produced faster than traditional manufacturing methods as development can begin as soon as the genetic sequence of a pathogen is available. Genetic vaccines are only capable of producing proteins and are unable to modify the host’s (vaccine recipient) own genetic material (DNA or mRNA).
Viral vector vaccines
Non-replicating and replicating viral vector vaccines are types of genetic vaccines. They work by using a modified virus (viral vector), which doesn’t cause disease in humans, to carry a portion of the pathogen’s genetic code into human cells.
In non-replicating viral vector vaccines, the individual’s cells will then produce proteins (antigens) specific to the pathogen to trigger an immune response. The COVID-19 vaccine Vaxzevria (AstraZeneca) is an example of a non-replicating viral vector vaccine.
Replicating viral vector vaccines have a similar mechanism but will also create new viral particles to enter further human cells, allowing a quicker and stronger immune response. Replicating viral vector vaccines best mimic natural infection and hence produce a strong immune response and can be used in lower doses.
While viral vectors are well tolerated and highly immunogenic in most people, pre-existing immunity to the viral vector used may hamper the immune response to the vaccine.
Nanoparticle-based vaccines
Nanoparticle-based vaccines have received increasing interest in recent years due to their good safety profile and high immunogenic potential.
Nanoparticle vaccines are constructed by attaching selected key components of a pathogen (such as the COVID-19 spike protein or viral DNA) to an engineered nanoparticle (nanocarrier). This nanoparticle is commonly an engineered virus-like particle (a molecule that mimics the virus but is not infectious). These nanoparticles are highly stable and less prone to degradation than traditional protein vaccines. Gardasil 9 (human papillomavirus) and Nuvaxovid (Novavax COVID-19) vaccines are examples of this approach.
资源
- Australian Immunisation Handbook: Types of vaccines
- Children’s Hospital of Philadelphia, Vaccine Education Centre: Making vaccines: How are vaccines made
- Frontiers in Immunology: Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines
作者: Daniela Say(MVEC 免疫研究员)和 Nigel Crawford(默多克儿童研究所 SAEFVIC 主任)
审核人: Rachael McGuire(MVEC 教育护士协调员)和 Francesca Machingaifa(MVEC 教育护士协调员)
日期: 4 月 1, 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
维多利亚州议会免疫会议
整个维多利亚州的每个地方议会都为所有受资助的国家免疫计划 (NIP) 疫苗的管理提供定期免疫会议。会议免费参加,需要 NIP 疫苗的所有年龄段的人都可以接种疫苗。会议由经过认证的护士免疫接种员在医疗支持下进行。一些委员会还可以选择为那些不符合资助疫苗资格标准的人购买额外的疫苗,例如脑膜炎球菌(Nimenrix® 或 Bexsero®)、流感和水痘疫苗 [参见资源]。所有接种的疫苗都记录在 澳大利亚免疫登记 (AIR).
请参阅下表了解您当地议会的联系方式,以获取有关会议时间的更多信息。
大都会议会
| 理事会 | 电话号码 | 免疫课程 |
|---|---|---|
| Banyule市议会 | (03) 9490 4222 | Banyule 免疫接种时间 |
| 贝赛德市议会 | (03) 9599 4444 | Bayside 免疫接种时间 |
| 布伦达拉市议会 | (03) 9278 4444 | Boroondara 免疫接种时间 |
| 布林班克市议会 | (03) 9248 4000 | Brimbank 免疫接种时间 |
| 卡迪尼亚郡议会 | 1300 787 624 | Cardinia 免疫接种时间 |
| 凯西市 | (03) 9705 5200 | 凯西免疫接种时间 |
| 戴尔宾市议会 | (03) 8470 8562 | Darebin 免疫接种时间 |
| 弗兰克斯顿市议会 | 1300 322 322 | 弗兰克斯顿免疫接种时间 |
| 格伦埃拉市议会 | (03) 9524 3333 | 格伦埃拉免疫接种时间 |
| 大丹德农市议会 | (03) 8571 1000 | 丹德农免疫接种时间 |
| 霍布森湾市议会 | (03) 9932 1000 | 霍布森湾免疫接种时间 |
| 休姆市议会 | (03) 9205 2200 | 休谟免疫接种时间 |
| 金斯顿市议会 | 1300 653 356 | 金士顿免疫接种时间 |
| 诺克斯市议会 | (03) 9298 8000 | 诺克斯免疫接种时间 |
| 曼宁厄姆市议会 | (03) 9840 9333 | Manningham 免疫接种时间 |
| 马瑞巴农市议会 | (03) 9688 0145 | Maribyrnong 免疫接种时间 |
| 马龙达市议会 | (03) 9298 4598 | Maroondah 免疫接种时间 |
| 墨尔本市议会 | (03) 9340 1451 | 墨尔本免疫接种时间 |
| 梅尔顿市议会 | (03) 9747 7200 | 梅尔顿免疫接种时间 |
| 蒙纳士市议会 | (03) 9518 3555 | 莫纳什免疫接种时间 |
| 满利谷市议会 | (03) 9243 8888 | 满利谷免疫接种时间 |
| 莫兰市议会 | (03) 9240 1111 | 莫兰德免疫接种时间 |
| 莫宁顿半岛郡 | (03) 5950 1000 | Mornington 免疫接种时间 |
| 尼伦比克郡议会 | (03) 9433 3111 | Nillumbik 免疫接种时间 |
| 菲利普港市议会 | (03) 9209 6383 | 菲利普港免疫接种时间 |
| 斯托宁顿市议会 | (03) 8290 1333 | Stonnington 免疫接种时间 |
| 白马市议会 | (03) 9262 6197 | 白马免疫接种时间 |
| 惠特尔西市议会 | (03) 9217 2170 | Whittlesea 免疫接种时间 |
| 温德姆市议会 | (03) 9742 0777 | 温德姆免疫接种时间 |
| 亚拉市议会 | (03) 9205 5555 | 雅拉市免疫接种时间 |
| 亚拉山脉郡议会 | 1300 368 333 | 亚拉山脉免疫接种时间 |
区域委员会
| 理事会 | 电话号码 | 免疫课程 |
|---|---|---|
| 阿尔卑斯郡议会 | (03) 5755 0555 | 高山免疫接种时间 |
| 亚拉腊乡镇议会 | (03) 5355 0200 | Ararat 免疫接种时间 |
| 巴拉瑞特市议会 | (03) 5320 5850 | 巴拉瑞特免疫接种时间 |
| 巴斯海岸郡议会 | (03) 5671 2211 | 巴斯海岸免疫接种时间 |
| Baw Baw 郡议会 | (03) 5624 2411 | Baw Baw 免疫接种时间 |
| 贝纳拉农村市议会 | (03) 5760 2600 | Benalla 免疫接种时间 |
| 布洛克郡议会 | 1300 520 520 | 布洛克免疫接种时间 |
| 坎帕斯佩郡议会 | (03) 5481 2200 | Campaspe 免疫接种时间 |
| 中央金矿区议会 | (03) 5461 0610 | Central Goldfields 免疫接种时间 |
| 科拉克奥特威郡议会 | (03) 5232 9400 | Colac-Otway 免疫接种时间 |
| Corangamite 郡议会 | (03) 5232 7100 | Corangamite 免疫接种时间 |
| 东吉普斯兰郡议会 | (03) 5153 9500 | 东吉普斯兰免疫接种时间 |
| 甘纳瓦拉郡议会 | (03) 5450 9333 | Gannawarra 免疫接种时间 |
| 格雷尔郡议会 | (03) 5522 2211 | Glenelg 免疫接种时间 |
| 黄金平原郡议会 | (03) 5220 7111 | Golden Plains 免疫接种时间 |
| 大本迪戈市议会 | (03) 5434 6000 | 本迪戈免疫接种时间 |
| 大吉朗市 | (03) 4215 6962 | 吉朗免疫接种时间 |
| 大谢珀顿市议会 | (03) 5832 9700 | Shepparton 免疫接种时间 |
| 赫本郡议会 | (03) 53482306 | 赫本免疫课次数 |
| 欣德马什郡议会 | (03) 5391 4444 | Hindmarsh 免疫接种时间 |
| 霍舍姆农村市议会 | (03) 5382 9777 | 霍舍姆免疫接种时间 |
| 靛蓝郡议会 | 1300 365 003 | 靛蓝免疫接种时间 |
| 拉特罗布市议会 | 1300 367 700 | 拉特罗布免疫接种时间 |
| 洛登郡议会 | (03) 5494 1200 | 洛登免疫接种时间 |
| 马其顿山脉郡议会 | (03) 5422 0333 | 马其顿山脉免疫接种时间 |
| 曼斯菲尔德郡议会 | (03) 5775 8555 | 曼斯菲尔德免疫会议时间 |
| 米尔迪拉农村市议会 | (03) 5018 8100 | 米尔杜拉免疫接种时间 |
| 米切尔郡议会 | (03) 5734 6200 | 米切尔免疫接种时间 |
| 莫伊拉郡议会 | (03) 5871 9222 | 莫伊拉免疫接种时间 |
| 穆拉布尔郡议会 | (03) 5366 7100 | Moorabool 免疫接种时间 |
| 亚历山大山郡议会 | (03) 5472 1364 | 亚历山大山免疫接种时间 |
| 莫恩郡议会 | 1300 656 564 | 莫因免疫接种时间 |
| 穆林丁迪郡议会 | (03) 5772 0333 | Murrindindi 免疫接种时间 |
| 北格兰屏郡议会 | (03) 5358 8700 | 北格兰屏免疫接种时间 |
| 比利牛斯郡议会 | (03) 5349 1100 | 比利牛斯山脉免疫接种时间 |
| 南吉普斯兰郡议会 | (03) 5662 9200 | 南吉普斯兰免疫接种时间 |
| 南格兰屏郡议会 | (03) 5573 0444 | 南格兰屏免疫接种时间 |
| 斯特拉博吉郡议会 | (03) 5795 0000 | Strathbogie 免疫接种时间 |
| 冲浪海岸郡议会 | 1300 610 600 | 冲浪海岸免疫接种时间 |
| 天鹅山农村市议会 | (03) 5036 2333 | Swan Hill 免疫接种时间 |
| 托旺郡议会 | (02) 6071 5100 | Towong 免疫接种时间 |
| 旺加拉塔农村城市 | (03) 5722 0888 | 旺加拉塔免疫接种时间 |
| 瓦南布尔市议会 | (03) 5559 4855 | 瓦南布尔免疫接种时间 |
| 惠灵顿郡议会 | 1300 366 244 | 惠灵顿免疫接种时间 |
| 西威默拉郡议会 | (03) 5585 9900 | West Wimmera 免疫接种时间 |
| 沃东加市 | (02) 6022 9300 | 沃东加免疫接种时间 |
| Yarriambiack 郡议会 | (03) 5398 0100 | Yarriambiack 免疫接种时间 |
有关此页面的任何反馈,请 在这里联系我们.
资源
作者: Rachael McGuire(默多克儿童研究所 SAEFVIC 研究护士)
审核人: Francesca Machingaifa(MVEC 教育护士协调员)
日期: 2022 年 2 月
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
疫苗信心
At MVEC we strongly encourage people to seek answers to their questions and to be well informed with evidence-based information. We know that nearly half of all parents have some concerns about immunising their children, ranging from minor concerns to more serious degrees of vaccine hesitancy. Many people also have questions about COVID-19 vaccines for themselves and their families.
There is a lot of information available to people, particularly on the internet, which can be quite overwhelming. There is also a lot of misinformation, and conspiracy theories, and it can be hard to know which information sources to trust and what is true. Research suggests that information alone is not enough to address people’s concerns about immunisation, even when it comes from recommended sources. That is because vaccine conversations matter, and how we discuss vaccines is often just as important as what information is shared.
Talking to people who have questions about vaccines
One of the most effective strategies to address people’s questions and concerns about vaccines is through a discussion with a trusted health care provider like a GP, nurse, paediatrician or midwife. Effective conversations are non-judgemental and help guide people towards accepting vaccination.
Having a combative conversation with a person who has questions about vaccines is never helpful. If possible, try to work out where the person may sit on the vaccine hesitency spectrum. They may have only minor concerns, more serious concerns or they may refuse vaccines all together. This often becomes apparent quite quickly as you start the conversation and helps you tailor your conversation accordingly.
Here are the key steps to have an effective vaccine conversation:
1. Find out all of the person’s questions and concerns
- Start with an open-ended question, like “What concerns do you have?”
- Try to just listen and not jump in and correct their beliefs straight away. This is what we call “resisting the righting reflex”
- Encourage them to share all their concerns before you start responding. They may even mention their most important concern last.
- At this point, once they have had a chance to list their concerns, summarise them to check your understanding.
2. Acknowledge concerns and share knowledge
- Not everyone is “vaccine hesitant”. Having questions is very normal, especially with the newer COVID-19 vaccines. People are likely to be more receptive to what you have to say if you acknowledge their concerns without judgement.
- It is helpful to ask if you can share what you know about vaccine safety and effectiveness and provide some good resources and information. Try to keep your explanations clear and check for understanding.
- At this point, it is good to reinforce their motivation to accept vaccination.
3. Discuss disease severity
- It is always good to bring the discussion back to centre on disease severity, rather than focusing exclusively on the vaccines. This reminds people why we are vaccinating and reinforces the benefit.
4. Recommend vaccination
- Lastly, make a clear and strong recommendation to have the vaccine(s). This reinforces the importance of vaccination and clearly shows that you believe this is the best way to protect the person against vaccine preventable diseases.
- If it is possible and the person is willing, deliver the vaccine(s) or explain where they need to go to receive them.
5. Continue the conversation
- If the person is not yet ready to accept vaccination, keep the communication open and invite them back at a later time to continue the conversation.
Talking with friends and family can also have an influence on people’s vaccine hesitancy. If you’re someone who wants to encourage others to vaccinate, you can take some of the strategies we recommend for healthcare providers into your discussions with people in your life.
This seems obvious, but the best approach is not to judge people, correct them, or jump into battle. This just entrenches people’s beliefs and makes them defensive. And they probably won’t trust you or want to talk to you openly about this topic anymore!
How to tackle misinformation
We’ve all heard people spreading misinformation and myths about vaccines. While it can be tempting to try to correct misinformation whenever you hear it, this can actually give the issue more oxygen. But if you notice that misinformation is spreading widely and beginning to affect people’s vaccination behaviour, it may be time to step in.
If an individual is spreading misinformation, try to speak with them privately. It’s not effective to have a public debate, either in person or online. Acknowledge the emotion and try to look for the truth together.
If you are debunking a particular myth, start by clearly restating the truth. Then, explain why the myth is untrue, and provide an alternative explanation for what the person is experiencing.
For example, if someone believes that the flu vaccine gives them the flu because they feel sick after the vaccine, it’s not enough to simply tell them that is untrue. Explain why this is untrue – the vaccine contains a killed virus that cannot cause the flu. Then follow this with an alternative explanation for the person’s symptoms – this is your body generating an immune response to the vaccine, and these symptoms are much more mild and brief than actual flu symptoms.
Finally, end by restating the truth. People remember what we say first and last, and what we say more than once. Make sure it’s the truth and not the myth that sticks in their minds.
资源
Talking to people who have concerns
- NCIRS Sharing Knowledge About Immunisation (SKAI) project
- MumBubVax: Talking about immunisation for mothers and babies
- The Conversation: Everyone can be effective advocate for vaccination- here’s how
Communicating about COVID-19 vaccines and vaccine safety
- World Health Organization: COVID-19 vaccines- safety surveillance manual
- Pre print MJA: Communicating with patients and the public about COVID-19 vaccine safety: recommendations from the Collaboration on Social Science in Immunisation
- COSSI: A COVID-19 vaccine strategy to support uptake amongst Australians: Working Paper November 2020
- ABC news: How to talk to friends and family feeling unsure about COVID-19 vaccines
- The COVID-19 Vaccine Communication Handbook
Addressing misinformation or talking about vaccination in online forums
- World Health Organization: How to respond to vocal vaccine deniers in public
- BMC Public Health: How organisations promoting vaccination respond to misinformation on social media: a qualitative investigation
- UNICEF: Vaccine misinformation management guide
- Tips on countering conspiracy theories and misinformation
作者: Margie Danchin (Senior Research Fellow, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
审核人: Margie Danchin (Group Leader, Vaccine Uptake Group, Murdoch Children’s Research Institute) and Jess Kaufman (Research Fellow, Vaccine Uptake Group, Murdoch Children’s Research Institute)
日期: June 2021
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
水痘
什么是流行性感冒?
Varicella (chickenpox) is a highly contagious disease caused by infection with the varicella-zoster virus (VZV). After a person recovers, the virus stays dormant (inactive) within the dorsal root ganglia of the spinal nerves and can later be reactivated, presenting as herpes zoster (shingles).
流感症状
Symptoms begin 7 to 21 days after exposure to the virus. Fever, rhinorrhoea (runny nose) and lethargy lasting 1 to 2 days are the first signs of disease (prodromal period). This is followed by the appearance of a pruritic (itchy) rash which is initially maculopapular (flat discoloured areas and raised areas) then becomes vesicular (fluid-filled blisters). The vesicles rupture then crust over, usually within 10 to 14 days. The rash can cover any part of the body including inside the mouth, on the scalp, eyelids and genital area. Unvaccinated children may experience 200 to 500 lesions. Approximately 5% of cases will be subclinical (symptoms are so mild they remain undetected under clinical examination).
Most cases of varicella are self-limiting (will resolve by itself), but complications can include secondary bacterial infections, septicaemia (blood infections), pneumonia, meningitis, encephalitis and acute cerebellar ataxia (uncoordinated muscle movements).
Varicella infections during pregnancy can result in congenital varicella syndrome in the child. Symptoms of congenital varicella syndrome include skin scarring, eye anomalies, limb defects and neurological malformations. The risk of congenital varicella syndrome is higher when infection occurs in the second trimester. Infants who have been exposed to varicella through a maternal varicella infection can develop herpes zoster, but this is rare.
Despite vaccination, some people may still develop disease if exposed to infection. This is known as breakthrough disease. Breakthrough varicella symptoms are usually mild. Patients are typically afebrile and develop fewer skin lesions. People experiencing breakthrough disease are contagious and can transmit disease to others.
结核病是如何传播的?
Varicella is transmitted by inhaling respiratory droplets that are made airborne when an infected person coughs or sneezes. It can also be spread by direct contact with the fluid inside the vesicles or through contact with items that have been contaminated with vesicle fluid.
A person with varicella is considered infectious for 1 to 2 days prior to the onset of the rash until all the lesions have crusted.
流行病学
Varicella is highly infectious. More than 80% of non-immune household contacts of an infected individual will develop disease.
Prior to the introduction of vaccination in Australia in 2005, there were 240,000 cases of varicella annually, resulting in 1,500 hospitalisations and an average of 7 to 8 deaths. The rate of hospitalisation for varicella infection was reduced to 2.1 per 100,000 people in 2013. Most of these children were aged under 18 months, the scheduled age for varicella vaccination. (NB: Children can be safely vaccinated from 9 months of age.)
Infants less than 4 weeks of age, premature neonates, unimmunised adolescents, 孕 women and immunosuppressed individuals are at 这 greatest risk of severe disease and complications if they become infected.
预防措施
Vaccines can provide protection against varicella infection.
| 年龄阶层 | Vaccine brand, antigen, dose and route | ||
| Varilrix (varicella) 0.5 mL SC | Varivax (varicella) 0.5 mL SC | Priorix Tetra (measles, mumps, rubella, varicella) 0.5 mL SC | |
| < 9 months | |||
| ≥ 9 months to < 12 months | ✓ | ✓ | |
| ≥ 12 months to < 12 years | ✓ | ✓ | ✓^ |
| ≥ 12 years | ✓ | ✓ | ✓ |
^ MMRV combination vaccines must not be given as the first dose of measles-containing vaccine in children < 4 years due to the higher rates of fevers (see precautions and contraindications below).
* Whilst not registered for use in those aged ≥ 12 years, ATAGI recommends it may be used up to 14 years. MVEC advises that its use may also be considered in older adults when protection against measles, mumps and rubella is also required.
shaded boxes – varicella vaccine is routinely offered on the NIP as a single dose at 18 months. AIR will consider vaccination from 12 months of age as a valid dose.
阴影方框表示未注册用于该年龄组别
shaded boxes indicate live-attenuated vaccines.
建议
A single dose of varicella vaccination (in combination with measles, mumps and rubella) is funded on the NIP at 18 months of age. (NB: Children can be safely vaccinated from 9 months of age.)
A second dose (not funded) is recommended for greater protection to reduce the incidence of breakthrough disease. A minimum interval of 4 weeks is recommended between doses of live-attenuated vaccines.
Anyone non-immune aged 14 years and over should receive a catch-up course of 2 doses (administered 4 weeks apart) for adequate protection.
Household contacts of 免疫功能低下 individuals are recommended to complete their immunisation schedule on time.
Common vaccine side effects
注射部位反应 (occurring in the first 48 hours) and fever (occurring 5 to 12 days after vaccination) are common side effects.
A maculopapular or vesicular rash (with 2 to 5 lesions) occurring 5 to 26 days after vaccination will develop in approximately 5% of vaccine recipients. If this occurs, it is recommended that lesions should remain covered until they crust over to avoid potential transmission of the virus to others. Transmission of the vaccine virus to contacts is extremely rare. Worldwide there have only been 11 documented cases of virus transmission from a recently vaccinated person to unvaccinated individuals.
Precautions and contraindications
All varicella vaccines are live-attenuated vaccines and are therefore contraindicated in pregnancy and in those with immunocompromise.
Children and adults who have been diagnosed with IFNAR1 should seek advice from an immunisation specialist or immunologist prior to vaccination.
There are recommended intervals between immunoglobulins or other blood products and administration of injected live-attenuated vaccines. Refer to MVEC:减毒活疫苗和免疫球蛋白或血液制品.
When administered as the first dose of an MMR-containing vaccine, MMRV 疫苗秒 have been associated with higher rates of fever in children. It is therefore recommended that MMRV vaccines are administered as the second dose only of the 2-dose course of MMR in children under 4 years of age.
暴露后预防
Vaccination (first or second dose) can be provided within 3 to 5 days of exposure to varicella (provided it is not contraindicated). This can reduce the likelihood of varicella disease developing.
Neonates (whose mother develops infection up to 7 days prior to delivery or within 2 days after delivery), infants under 1 month of age (if mother is seronegative), pregnant women, premature infants (while still hospitalised, regardless of maternal serology) or immunosuppressed individuals who are exposed to varicella disease should receive Zoster Immunoglobulin (ZIG).
Repeat doses of ZIG may be given if a person is exposed to varicella again more than 3 weeks after the first dose of ZIG.
资源
- RCH Kids health information: Varicella fact sheet
- Australian Immunisation Handbook: Varicella
- MVEC:带状疱疹
- MVEC:减毒活疫苗和免疫球蛋白或血液制品
作者: Rachael McGuire (Research Nurse, SAEFVIC, Murdoch Children’s Research Institute)
审核人: Rachael McGuire (MVEC Education Nurse Coordinator) and Katie Butler (MVEC Education Nurse Coordinator)
日期: October 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.