Background
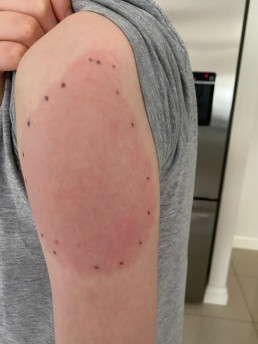
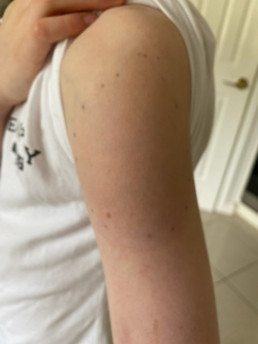
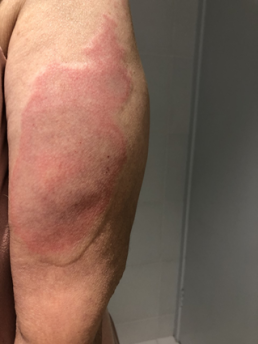
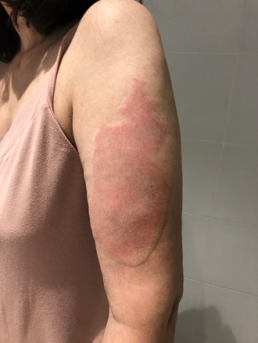
Injection site reactions (ISRs) are a common localised side effect that can occur following the administration of any injected vaccine. They are an inflammatory response to the injected vaccine. Symptoms of ISRs include swelling, redness (erythema), induration (hardness), pain or itch at or near the injection site.
Less commonly, significant or severe ISRs can present after an injection, extending over a greater area from ‘joint-to-joint’ (e.g. from the shoulder joint to the elbow joint) or ‘crossing joints’ (e.g. swelling that passes over one joint, such as the shoulder or the knee).
High-grade fevers are not typically associated with ISRs.
Diagnosis
A GP, immunisation specialist or immunisation provider can diagnose an ISR based on the clinical symptoms and timeline of presentation.
ISRs typically occur within the first 48 hours following vaccination. Symptoms usually last 1 to 2 days and completely resolve within a week. In some rarer instances ISRs can last for a longer period of time (e.g. 5 to 7 days) or have a delayed onset (3 or more days after vaccination).
Photographic evidence and monitoring the circumference of the affected area over time (e.g. draw around the ISR with a pen to see changes in circumference) can assist in documenting the progression of symptoms and support a diagnosis.
Significant ISRs should not be confused with infections such as cellulitis. Symptoms consistent with cellulitis, such as decreased range of movement, lymphangitis (tracking of erythema), lymphadenopathy (swollen lymph nodes) and high-grade fevers, are not consistent with ISRs.
ISRs are not an allergic response.
Association and incidence
The true incidence of ISRs is difficult to ascertain as they are often underreported. All injected vaccines have the potential to cause ISRs. However, factors such as the inclusion of adjuvants in the vaccine ingredients, previous exposure to an antigen (through vaccination) and the injection technique used can influence the likelihood of an ISR developing.
Vaccines that contain adjuvants (specific ingredients that are included to evoke stronger immune responses) are often associated with a higher incidence of ISRs. Examples of these vaccines include Shingrix (vaccination against the development of herpes zoster), tetanus-containing vaccines (such as Infanrix hexa and Boostrix), meningococcal vaccines (such as Bexsero), pneumococcal vaccines (Pneumovax 23 and Prevenar 13) and adjuvanted influenza vaccines given to those aged 65 years and over (Fluad Quad and Fluzone High-Dose Quadrivalent).
COVID-19 vaccines and Prevenar 13 are associated with delayed-onset ISRs (occurring more than 3 days after vaccination) when administered to adults 70 years and older.
There is an increased likelihood of ISRs occurring following subsequent doses (primary or boosters) of a particular antigen (e.g. in children aged 18 months and 4 years following diphtheria-tetanus-pertussis boosters, and those receiving subsequent doses of pneumococcal vaccines).
The inadvertent administration of a vaccine into the subcutaneous (SC) tissue, where intramuscular (IM) administration is recommended, has also been associated with an increased incidence of ISR development. Correct injection technique (including route, and appropriate needle size and length) plays an important in mitigating the development of ISRs.
Treatment
ISRs will resolve on their own without intervention. They can generally be managed at home with symptomatic relief such as oral analgesia and applying a cold compress to the affected area. Immobilising the affected limb should be avoided; movement will enhance and assist with lymphatic drainage to improve symptoms.
ISRs are not a sign of allergy or local infection. Therefore, antihistamines, steroids or antibiotics are not required.
Implications for future doses
While ISRs are a common and expected side effect following most injected vaccines, significant or severe ISRs should be reported to SAEFVIC in Victoria.
A previous experience of an ISR is not a contraindication to future doses of the same or any other vaccine. Individuals who have experienced ISRs in the past may experience them again, but they are unlikely to be worse. The benefit of being protected against vaccine-preventable diseases far outweighs the possible risk of developing an ISR. Vaccine recipients who have previously experienced an ISR are encouraged to complete the recommended vaccine schedules.
Resources
- MVEC: Adverse Event Reporting Australia
- MVEC: SAEFVIC
- MVEC: Injection site nodules
- Australian Immunisation Handbook: Common AEFIs and how to manage them
- Australian Immunisation Handbook: Table. Comparison of the effects of diseases and the side effects of vaccines on the NIP
- Australian Immunisation Handbook: Vaccination procedures. After vaccination
- NCIRS: Injection site reactions information sheet
Author: Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Melissa Humann (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Rachael McGuire (MVEC Education Nurse Coordinator) and Katie Butler (MVEC Education Nurse Coordinator)
Date: December 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.