Specialist Immunisation Services
Background
Specialist immunisation services (SIS) are hospital-based immunisation clinics led by a team of specialised medical and nursing staff with expertise in vaccines and immunisation.
In Victoria, there are specialised immunisation services at Monash Health, Western Health, Royal Children’s Hospital and Alfred Health.
Purpose of SIS
These services offer vaccination encounters through walk-ins, or via formal appointments for patients with more complex vaccination needs (e.g. multiple medical conditions, antenatal vaccination, complex catch-up plans, oncology, pre- and post-transplant recipients).
SIS in Victoria are also linked with SAEFVIC, the Victorian vaccine safety service. Specialist consultation and/or vaccination under supervision may be facilitated via these services for individuals who have previously experienced a significant adverse event following immunisation (AEFI) or who are at higher risk of experiencing an AEFI.
Access and services offered
Royal Children’s Hospital Immunisation Service
Suited for:
Children of all ages and family members (*BCG appointments only available for children < 12 months of age)Services offered:
NIP and travel vaccines, catch-up vaccination (appointments required), COVID-19 vaccines (from 6 months of age), meningococcal B/ACWY, sedation services and complex vaccination needsHours of operation:
Monday to Friday 9:00 am–4:30 pm, excluding public holidaysHow to access:
Walk-ins and appointments availableHow to refer:
Immunisation clinic fax number: (03) 9345 4163
BCG clinic fax number: (03) 9345 5034
Inpatients/outpatients (seen by Immunisation Nurse Practitioners) fax number: (03) 9345 4100Location:
Ground floor opposite Parkville Café, Royal Children’s Hospital, ParkvilleContact:
Telephone: 1300 882 924 (option 2) or (03) 9345 6599 / 9345 6399
Email: [email protected]Monash Health Immunisation Service
Suited for:
All ages (*BCG appointments only available for children < 5 years of age)Services offered:
National Immunisation Program (NIP) and travel vaccines, catch-up plans and vaccination, sedation services, complex vaccination needs and COVID-19 vaccines (≥ 6 months)Hours of operation:
Monday to Friday 8:30 am–4:00 pm, excluding public holidays.How to access:
Walk-ins and appointmentsLocation:
Suite I, Jessie McPherson Private Consulting Suite Level 2, Monash Medical Centre, ClaytonHow to refer:
Monash Health require all referrals to be submitted from your GP via HealthLink: Monash Health referralsContact:
Telephone: (03) 9594 6320
Email: [email protected]Western Health Immunisation Service
Suited for:
Children and pregnant peopleServices offered:
NIP vaccines (children), antenatal vaccines (dTpa and influenza) and COVID-19 vaccines (≥ 6 months)Hours of operation:
Monday to Friday 9:00 am–4:00 pm, excluding public holidaysHow to access:
Walk-ins and appointments (appointments held on Wednesday afternoons only)Location:
Ground Floor, Sunshine Hospital, Joan Kirner Women and Children’s Hospital, St AlbansHow to refer:
Western Health require all external patients to be referral through the general paediatric referral system: Western Health ReferralContact:
Telephone: (03) 8345 1727
Email: [email protected]Alfred Health Specialist Immunisation Services (AHSIS)
Suited for:
Adults only (≥ 18 years)Services offered:
Individuals who have experienced AEFI or who are at risk of experiencing AEFIHow to access:
By appointment onlyHow to refer:
Referrals can be made to Alfred Health Specialist Clinics and sent via Fax: (03) 90766938 or Email: [email protected]Contact:
Telephone: (03) 90765200
Funding
The Royal Children’s Hospital, Monash and Western Health services operate with funding from the Victorian Department of Health. Alfred Health Specialist Immunisation Service is fully funded internally by Alfred Health.
Resources
- MVEC: SAEFVIC
- MVEC: Needle phobia
- MVEC: Meningococcal
- MVEC: Asplenia and hyposplenia
- MVEC: Cancer immunisation guideline
- MVEC: Solid organ transplant
- MVEC: BCG/Tuberculosis
- MVEC: Influenza
Authors: Adele Harris (Immunisation nurse, SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: March 2024
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Surveillance of Adverse Events Following Vaccination In the Community (SAEFVIC)
SAEFVIC (Surveillance of Adverse Events Following Vaccination In the Community) is the central reporting service in Victoria for any significant adverse event following immunisation (AEFI).
An AEFI is defined by the Australian Immunisation Handbook as “any untoward medical occurrence that follows immunisation. It does not necessarily have a causal relationship with the vaccine”. A vaccine error is also considered an AEFI and may be related to the way a vaccine was stored, prepared or administered.
Reporting adverse events is not mandatory in Victoria, however doing so allows the rapid investigation of any potential vaccine or system problems by Victorian and national health authorities (Therapeutic Goods Administration). This helps to ensure a safe and effective immunisation program and it maintains community confidence in vaccines.
Following the report of adverse events, SAEFVIC can facilitate individualised clinical assistance for patients and families affected by an AEFI. This may be done via a face-to-face or telehealth consultation with a specialist or with an immunisation nurse over the phone.
Please see your GP, local emergency department or call 000 if immediate assistance is required.
Resources
- SAEFVIC
- MVEC: Victorian Specialist Immunisation Services (VicSIS)
- MVEC: Adverse events reporting Australia
- MVEC: AusVaxSafety: vaccine safety surveillance in Australia
Authors: Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute), Georgina Lewis (Clinical Manager, SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (Research Nurse, SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: October 20, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Shoulder injury related to vaccine administration (SIRVA)
What is it?
Shoulder Injury Related to Vaccine Administration (SIRVA) is a rare but serious complication following suspected inadvertent administration of a vaccine too high in the deltoid or into the shoulder joint. This may cause a local inflammatory response and potential trauma to local structures within the shoulder joint including bursae, ligaments and tendons resulting in sudden onset shoulder pain and restricted movement. Symptoms can last for weeks to months or as long as years. Affected individuals can experience varying degrees of disability which can impact on their activities of daily living, social and emotional wellbeing.
Symptoms
Distinguishing symptoms/features of SIRVA include:
- sudden onset shoulder pain within 48 hours of vaccination- different to the injection site pain expected following vaccination
- restricted range of movement (RROM) of affected shoulder
- persistent shoulder pain and RROM lasting >1 week, lasting weeks to months
- suspicion of incorrect vaccination site – too high in the upper arm.
Impacts and implications
The impacts of SIRVA can include:
- interrupted sleep due to pain
- difficulty with personal care, care of others and activities of daily living
- inability to participate in sports or hobbies
- modified work duties
- time off work related to symptoms and/or treatments and investigations
- loss of income due to time off work
- financial burden due to cost of treatments and investigations
- emotional and social wellbeing.
Further implications for an individual with SIRVA can include vaccine hesitancy, reduced confidence in healthcare/immunisation providers and the potential for impaired immunogenicity.
Diagnosis
A GP, specialist or allied health professional such as a physiotherapist can diagnose SIRVA based on presenting symptoms and clinical history following an immunisation.
If radiological investigations such as ultrasound or MRI are undertaken to support or confirm a diagnosis, abnormalities including bursitis, adhesive capsulitis, impingement syndrome, synovitis or tendon tears may be identified.
Early diagnosis of SIRVA leads to timely treatment which is thought to lessen the duration and severity of symptoms.
Treatment options
SIRVA can be treated in a variety of ways and may include any of the following:
- over the counter pain/anti-inflammatory medications
- prescription pain/anti-inflammatory medication
- oral corticosteroids
- corticosteroid joint injections
- physiotherapy or other allied health professionals
- massage
- surgery(rare).
How to prevent SIRVA
SIRVA can be prevented by following the recommended vaccination procedures for correct injection technique.
| Expose | Identify | Imagine | Inject |
| Expose the whole upper arm | Identify upper and lower anatomical landmarks (acromion and deltoid tuberosity) | Imagine an inverted triangle 2-3 fingers below the acromion | Inject vaccine in the centre of the triangle into the deltoid muscle |
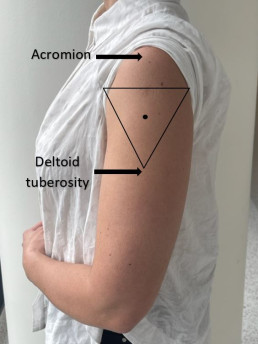
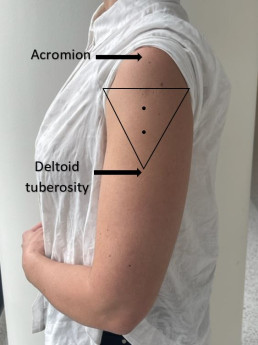
Please refer to MVEC: Administration of injected vaccines- correct technique for further information on correct injection technique.
Where to report a case of SIRVA
All confirmed or suspected cases of SIRVA should be reported to SAEFVIC (the Victorian vaccine safety service). Reports can be made by consumers, immunisation providers or treating healthcare professionals.
SAEFVIC can provide clinical advice or facilitate consultation with an immunisation specialist if required.
Resources
- MVEC: Administration of injected vaccines- correct technique
- MVEC Education portal eLearning package: Shoulder Injury Related to Vaccine Administration (SIRVA)
- Australian immunisation handbook: Avoiding shoulder injury related to vaccine administration
- SIRVA (Shoulder injury related to vaccine administration); A case series- “Are you on target?”
- Cross GB, Moghaddas J, Buttery J, Ayoub S, Korman TM. Don’t aim too high: Avoiding shoulder injury related to vaccine administration. Aust Fam Physician 2016;45(5):303-306
- Petrakis N, Addison M, Penak B, Schrader S, Mallard J, Clothier H. J , Buttery J. P, Crawford N. W & Cheng D. R (2023) Shoulder injury following COVID-19 vaccine administration: a case series and proposed diagnostic algorithm, Expert Review of Vaccines, 22:1, 299-306
Authors: Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Georgie Lewis (SAEFVIC Clinical Manager, Murdoch Children’s Research Institute) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Date: March 23, 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Special Risk Chapter in the Australian Immunisation Handbook
What is it?
Individuals that are at higher risk of vaccine preventable diseases (VPD) are classified as ‘special risk’ groups in the Australian Immunisation Handbook.
This includes populations at special risk (e.g. Aboriginal and Torres Straight Islanders) and those with additional vaccine requirements (e.g. maternal vaccination; preterm infants). It also has detailed sections on those at special risk because of immune suppression (disease and/or therapy) e.g. Asplenia, cancer/chemotherapy.
The chapter is updated online using the latest available scientific evidence
The Handbook is endorsed by:
- The Australian Technical Advisory Group on Immunisation [ATAGI] and
- The National Health and Medical Research Council [NHMRC]
Resources
- Australian Immunisation Handbook: Vaccination for Special Risk Groups
- MVEC: Australian Technical Advisory Group on Immunisation
Reviewed by: Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute)
Date: September 2018
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Solid organ transplant recipient: pre-transplant immunisation recommendations
Background
In order to prevent the rejection of transplanted organs, people who have undergone a solid organ transplant require varying doses of immune suppressive medication. Once a patient is immune suppressed, seroprotection gained from immunisation may be suboptimal and therefore additional doses of vaccines may be recommended. Some vaccines (live-attenuated vaccines) may be contraindicated.
To overcome this and maximise immune responses it is recommended where possible that all vaccines are administered well before transplant with live-attenuated vaccines administered a minimum of 4 weeks prior to transplant.
Please refer to MVEC: Pre-solid organ transplant recipient immunisation guideline (0-18 years) for more information.
For immunisation recommendations following a solid organ transplant please refer to your immunisation specialist for specific advice.
MVEC special risk guidelines
These guidelines have been prepared by immunisation staff from the Royal Children’s Hospital and Monash Health and endorsed at a monthly immunisation meeting. Attendees at this meeting include paediatricians, infectious disease physicians, nurse immunisation specialists, infection control team members and a representative from the Immunisation Section of the Victorian Department of Health.
These guidelines are based on the latest available evidence and aim to align with recommendations in the Australian Immunisation Handbook.
Vaccine funding
Some of the recommendations in these guidelines are outside the scope of the National Immunisation Program (NIP). Different jurisdictions and individual hospitals have varying approaches to non-NIP vaccines, which should be clarified with the local health service.
We welcome any feedback on the guidelines, please email: [email protected]
Resources
Authors: Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Annie Cobbledick (Immunisation Pharmacist, the Royal Children’s Hospital)
Date: March 2021
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.