Tuberculosis
What is it?
Tuberculosis (TB) is caused by an infection with the bacteria Mycobacterium tuberculosis. In rarer cases, TB may be attributed to infection with Mycobacterium bovis or Mycobacterium africanum.
TB is categorised as being an active disease (the infected person is displaying symptoms and is infectious) or a latent infection (the bacteria is dormant and the infected person has no symptoms and is not infectious). Latent TB can become active if an infected person’s immune system becomes weakened (due to a medical condition or taking immunosuppressive therapies), following serious illness, with drug or alcohol abuse, or with increasing age.
What to look for
Approximately 60% of TB cases in Australia present as pulmonary (lung) TB. Common symptoms include a chronic cough which may be accompanied by haemoptysis (coughing blood) as well as fevers, night sweats, weight loss and malaise (generally feeling unwell).
In some cases, infection can also occur in the brain, bones, kidneys or lymph nodes. TB meningitis (brain infection) and TB septicaemia (blood infection) are considered the most serious manifestations of TB disease.
Most people infected with TB are asymptomatic. 10% of people infected with TB will go on to develop clinical disease at some point in their lifetime.
How is it transmitted?
Pulmonary TB can be spread to others via aerosol transmission of infected droplets through coughing or sneezing. People with TB infections without lung or laryngeal involvement generally cannot transmit disease. However, urine is considered infectious in the case of renal TB. In countries where Mycobacterium bovis is prevalent (it is not prevalent in Australia), the bacteria can also be transmitted by ingesting unpasteurised milk.
A person is considered infectious until 14 days after being on active treatment AND until they have tested negative on a sputum sample. Following an active infection the bacteria will remain dormant (non-infectious) within scar tissue where it can be reactivated later in life (even decades later). Once reactivated the bacteria is capable of being transmitted again.
Epidemiology
Australia has one of the lowest rates of TB in the world, affecting less than 7 per 100,000 people. Aboriginal and Torres Strait Islander people residing in the Northern Territory and Far North Queensland have a much greater burden of disease compared with non-Indigenous people in those areas and Aboriginal and Torres Strait Islander people living in other states.
Globally, it is estimated that approximately one-quarter of the world’s population is infected with TB, causing 1.5 million deaths annually. Bangladesh, China, India, Indonesia, Nigeria, Pakistan, Philippines and South Africa have the highest incidence of infection, accounting for almost half of the world’s TB cases.
Children under 5 years of age, the older population, those with immunocompromise (due to medical condition or immunocompromising therapies) and those impacted by poor living conditions have the highest rates of infection.
Prevention
Vaccination with the BCG vaccine is effective in reducing the incidence of TB meningitis and death in children less than 5 years of age in countries where TB is prevalent. Vaccination is not routinely recommended in developed countries like Australia where disease incidence is low. In Australia the following groups of people are recommended to be vaccinated:
- children < 5 years living in Aboriginal and Torres Strait Islander communities in Queensland
- Aboriginal and Torres Strait Islander neonates living in Queensland
- children < 5 years of age travelling internationally to areas where tuberculosis is prevalent.
BCG vaccination for individuals travelling to areas with a high incidence of disease is ideally administered 4-6 weeks prior to travel.
Tuberculin skin testing
Tuberculin skin testing (TST) or Mantoux testing prior to BCG vaccination is recommended on a case-by case basis to determine if a person already has a level of immunity to TB. It involves the intradermal injection of a tuberculin purified protein derivative (PPD).
In people who have previously received a BCG or have previously had TB exposure, a hypersensitivity reaction can be recognised 48-72 hours later.
It is important to note that TST results may be unreliable for 4-6 weeks following a measles infection or receiving a measles-containing vaccine.
Vaccine administration
BCG vaccine is administered via the intradermal route. Only healthcare providers trained in intradermal technique should provide BCG vaccination.
The recommended site for injection is on the left arm over where the deltoid muscle inserts into the humerus. Administration at this site will minimise the risk of keloid scarring.
- In children < 12 months of age the recommended dose is 0.05ml
- In children > 12 months of age the recommended dose is 0.1ml
Other injected live-attenuated vaccines can either be administered on the same day as BCG or 4 weeks apart. There is no recommended interval between BCG and oral live-attenuated vaccines.
Contraindications and precautions
BCG is a live-attenuated vaccine and is therefore contraindicated in individuals with immunosuppression or in those who are pregnant.
If active eczema, dermatitis or psoriasis is present at the site of injection, vaccination should be deferred until the skin can be treated and is clear of symptoms.
Side effects following vaccination
BCG, like all vaccines, has a list of common and expected side effects and a list of rare side effects that may occur in the weeks following vaccine administration.
Common and expected side effects can include:
- a small red papule will appear at the injection site in the weeks following the vaccine
- an ulcer (open sore) may develop 2-3 weeks later (usually less than 1 cm in diameter) and last from a few weeks to months
- the majority of infants will develop a flat scar at the site once the ulcer heals.
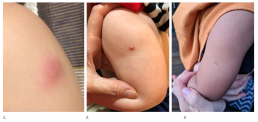
1. Papule
2. Ulcer
3. Scar
Rare or more serious side effects can include:
- axillary lymphadenopathy (swelling of the lymph nodes under the left arm)
- persisting ulcer lasting longer than a few months
- a large abscess (collection of pus) at the injection site
- keloid scarring at the site.
NB: If you suspect a rare or serious side effect, it is strongly recommended to seek medical advice either from a GP or the medical clinic where the BCG was administered. For specialist immunisation advice or to report an adverse event following immunisation (AEFI), please contact SAEFVIC.
Post vaccination care
Following vaccination the site must be kept clean and dry. Normal activities like bathing and swimming can continue ensuring that the area is patted dry afterwards.
If the ulcer is oozing it may be covered with a dry gauze and can be cleaned with an alcohol swab. Ointments, creams or antiseptics should not be applied directly to the site.
For more information refer to What to expect following the BCG vaccination – RCH parent handout.
Resources
BCG Clinics in Victoria
- The Royal Children’s Hospital: BCG Clinic
- Monash Health Immunisation Service
- Offspring Child Health Specialists
- Family Immunisation & Travel Specialists (FITS)
- A/Prof Mike Starr
- Craigieburn Specialist Consulting Suites
- Medical One- QV
- Kids Travel Doc
- The Children’s Private Medical Group
- Wyndham Private Medical Centre
- Travel Clinics Australia- Caulfied
- Pinnacle Medical Centre – Williams Landing
- The Travel Doctor – TMVC at Sonic HealthPlus CBD clinic
- CBD Doctors Melbourne
Other
- MVEC: Intradermal vaccination
- RCH Kids Health Information: Tuberculosis disease (TB)
- Australian Immunisation Handbook: Tuberculosis
- RCH Kids Health Information BCG vaccine for TB
- What to expect following the BCG vaccination – RCH parent handout
- What to expect after your child receives the BCG vaccination – Monash Hospital parent handout
- Dhanawade, S. Kumbhar, S. Gore, A. and Patil, V. Scar formation and tuberculin conversion following BCG vaccination in infants: A prospective cohort study, Journal of Family Medicine and Primary Care 2015 Jul-Sep 4(3) 384-387
Authors: Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: March 13, 2024
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Typhoid
What is it?
Typhoid and paratyphoid fever are caused by bacterial infections with the Salmonella enterica subspecies Typhi and Paratyphi (A, B and C). Collectively, typhoid and paratyphoid fever are known as enteric fever. Typhoid fever presents more commonly than paratyphoid fever and is associated with higher rates of severe complications and poorer outcomes. The similarities between typhoid and paratyphoid fever make it difficult to distinguish between the two diseases without diagnostic testing.
Enteric fever is different to the gastroenteritis that can be caused by other Salmonella bacteria.
What to look for
The incubation period of typhoid fever is usually 7 to 14 days but can range from 3 to 60 days. Typhoid and paratyphoid fever often present with prolonged fever and fatigue, headache, splenomegaly (enlargement of the spleen), abdominal symptoms (pain, lack of appetite, constipation or diarrhoea) and bacteraemia (bacteria in the bloodstream). A rash appearing as small pink clusters on the skin known as ‘rose spots’ can be seen in up to 30% of individuals with enteric fever. In severe cases, complications can include septic shock, gastrointestinal bleeding with perforation, altered conscious state. If left untreated, enteric fever can be fatal.
How is it transmitted?
Salmonella enterica is transmitted via the faecal–oral route and through ingestion of contaminated food and water sources. It is more common in developing countries with untreated drinking water and inadequate sanitation and food handling practices.
Approximately 10% of people infected with Salmonella enterica will excrete the bacteria for up to 3 months following acute infection. Approximately 1 in 20 infected individuals who do not receive treatment for typhoid fever will become an asymptomatic carrier of the disease.
Epidemiology
Although it is not prevalent in Australia, typhoid is a common disease in many parts of the world, especially Asia, southeast Asia and sub-Saharan Africa. In Australia, 100 to 200 returned travellers are diagnosed with typhoid every year
In 2019 it was estimated that 9 million cases of typhoid occur globally each year, resulting in 110,000 deaths. Children are disproportionately affected by infection.
Prevention
Prevention of typhoid fever includes both vaccination and undertaking food and water precautions whilst travelling in developing countries where typhoid is endemic.
Food and water precautions
Travellers should maintain the following precautions to limit exposure to infections:
- undertake effective hand washing practices
- drink bottled or boiled water
- avoid ice ice unless it is made from safe water
- ensure food is properly cooked
- avoid raw milk and products made from raw milk
- wash fruits and vegetables carefully, particularly if they are eaten raw
- when possible, peel vegetables and fruits.
Vaccines
There are 3 vaccines available in Australia for protection against typhoid fever:
| Age group | Vaccine brand, antigen, dose and route | Primary course | Booster |
| ≥ 2 years | Typhim Vi (typhoid) 0.5 mL IM | 1 dose | after 3 years |
| ≥ 6 years | Vivotif (typhoid*) 1 capsule oral | 3 doses (day 1, 3 and 5) | after 3 years |
| 4 doses (day 1, 3, 5 and 7) | after 4 years | ||
| ≥ 16 years | Vivaxim (typhoid, hepatitis A) 1.0 mL IM | 2 doses (6 months apart) | typhoid only after 3 years (hepatitis A booster not required) |
Blue shading = live-attenuated vaccine
§ While Vivaxim is not registered for use in children aged under 16 years, some immunisation specialists and travel medicine practitioners administer Vivaxim to children aged 2–15 years. Refer to Lau C. et al The tolerability of a combined hepatitis A and typhoid vaccine in children aged 2-16 years: an observational study Journal of Travel Medicine 2016: 15, 23 (2) for more information.
* There is no specific vaccine for paratyphoid fever however there is some evidence to suggest that administration of the oral typhoid fever vaccine (Vivotif) can provide some cross–protection against paratyphoid fever.
Precautions and contraindications
People taking both the oral typhoid vaccine (Vivotif) and the oral cholera vaccine (Dukoral) should separate these 2 vaccines by 8 hours, due to the risk of components of the cholera vaccine impacting how the typhoid vaccine is absorbed.
Vivotif is live-attenuated vaccine and is therefore contraindicated in pregnant people and in individuals with immunocompromise.
People who are currently receiving antibiotics, sulfonamides or antimalarial prophylaxis should receive one of the injected typhoid vaccines (Typhim Vi or Vivaxim) instead of Vivotif. Alternatively, time the last dose of the oral vaccine at least 3 days before commencing antibiotics, sulfonamides or antimalarial prophylaxis.
Vaccine side effects
Side effects from typhoid vaccination are usually mild and short lived.
Injection site reactions commonly occur in people who have received injected typhoid vaccines (Typhim Vi and Vivaxim). Abdominal pain, diarrhoea, nausea, vomiting and urticaria (itchy rash) commonly occur after vaccination with the oral typhoid vaccine (Vivotif).
Resources
- Australian Immunisation Handbook: Typhoid fever
- The blue book: guidelines for the control of infectious diseases
- World Health Organization: Typhoid
Authors: Rachael Purcell (RCH Immunisation Fellow), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator) and Katie Butler (MVEC Education Nurse Coordinator)
Date: November 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Thrombosis with thrombocytopenia syndrome (TTS)
Thrombosis with thrombocytopenia syndrome (TTS), also known as Vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) or vaccine-induced immune thrombotic thrombocytopenia (VITT), was a rare syndrome reported in people who received adenoviral vector COVID-19 vaccines such as Vaxzevria (AstraZeneca) and the Johnson & Johnson/Janssen COVID-19 vaccine.
Please note: adenoviral vector COVID-19 vaccines are no longer available for use in Australia. The following information remains for reference purposes.
What is TTS?
The syndrome is characterised by thrombosis formation (blood clots) combined with thrombocytopenia (low platelet count) with symptoms typically presenting in the 4-42 days after vaccination.
According to the Center for Diseases Control (CDC) TTS is classified into 2 tiers based on the location of thrombosis and severity of symptoms. Cases are further classified based on if there has been recent exposure to heparin or not.
Tier 1:
- uncommon site of thrombosis (eg. brain – cerebral venous sinus thrombosis [CVST] or gut – eg. splanchnic vein, associated with bowel ischaemia and surgery, portal vein or other rare venous and arterial thromboses)
- may also concurrently have thrombosis in more common locations (eg. deep vein thrombosis or pulmonary embolism)
- platelet count < 150,000 per microliter
- positive (+) anti-PF4 ELISA result is supportive, but not required for diagnosis.
Tier 2:
- common sites of thrombosis such as leg or lungs (eg. venous thromboembolism, deep vein thrombosis, pulmonary embolism)
- platelet count < 150,000 per microliter
- positive anti-PF4 ELISA result is required.
Tier 1 tends to be associated with more severe presentations and carries a higher risk of morbidity and mortality than Tier 2. Evidence suggests that Tier 1 is more common in younger age groups.
How did adenoviral vector vaccines trigger TTS?
The exact mechanism of how TTS is triggered is still under investigation, however the majority of cases were associated with the finding of anti-PF4 antibodies. There are no clear diagnostic markers to indicate who is at risk of this syndrome. This is currently under investigation.
TTS appears to be similar to an autoimmune condition known as heparin induced thrombocytopenia (HIT), which is where an immune reaction to the medication heparin impacts platelet function, leading to thrombosis (clots).
The risk of TTS
It is estimated that the risk of developing TTS after dose 1 of Vaxzevria (AstraZeneca) was approximately 2.6 per 100,000 persons, with those under 60 years experiencing more severe outcomes. The risk of developing TTS following dose 2 occurred at a much lower rate.
The risk of developing other clotting disorders after receiving adenviral vector vaccines
There is currently no evidence that adenoviral vector vaccines increase the overall risk of developing other standalone thromboses (eg. other clotting disorders leading to deep vein thromboses, pulmonary emboli, myocardial infarction, stroke) beyond the baseline rate in the general population.
TTS was different to other commonly diagnosed thromboses as it was triggered by an immune response to a specific type of vaccine which then results in the combination of both thrombosis (clots) and thrombocytopenia (low platelet count).
Resources
- MJA: Australian and New Zealand approach to diagnosis and management of vaccine‐induced immune thrombosis and thrombocytopenia
- Thrombosis and Haemostasis Society of Australia and New Zealand (THANZ) Multidisciplinary VITT Guideline for Doctors
- HealthPathways Thrombosis with Thrombocytopenia Syndrome (TTS) pathway (login required)
- ATAGI statement on revised recommendations on the use of COVID-19 Vaccine AstraZeneca, 17 June 2021
- ATAGI: Joint statement from ATAGI and THANZ on Thrombosis with Thrombocytopenia Syndrome (TTS) and the use of COVID-19 AstraZeneca
- THANZ advisory statement for haemotologists, August 19, 2021: Suspected Vaccine induced immune thrombotic thrombocytopenia (VITT)
- Australasian College of Emergency Medicine: Thrombosis with Thrombocytopenia Syndrome following COVID-19 vaccination
- COVID-19 vaccination – Information for Immunisation Providers on Thrombosis with Thrombocytopenia Syndrome (TTS) following COVID-19 vaccination
- Patient information sheet on AstraZeneca COVID-19 vaccine and thrombosis with thrombocytopenia syndrome (TTS)
Authors: Daryl Cheng (Paediatricican, Royal Children’s Hospital), Francesca Machingaifa (MVEC Education Nurse Coordinator), Davina Buntsma (MVEC Immunisation Fellow), Rachael McGuire (MVEC Education Nurse Coordinator), Linny Kimly Phuong (Immunisation Consultant, SAEFVIC) and Hannah Morgan (Epidemiologist, SAEFVIC)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: April 2024
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Tetanus
What is it?
Tetanus is caused by infection with the bacteria Clostridium Tetani. The bacteria secrete a potent exotoxin that enters the bloodstream, acting on the central nervous system to cause muscle rigidity and painful spasms. Tetanus can be fatal when the muscles controlling respiration are affected.
What to look for
The incubation period of tetanus is 3 to 21 days. The most common time frame for presentation of tetanus is around 14 days after exposure. Although, it can present as early as 1 day or as long as several months after the exposure.
The initial symptom is muscle rigidity. This can affect all muscles in the body but may be more pronounced in those closest to the site of the injury. In some cases, symptoms may include hyperreflexia (hyperactive reflexes) resulting in back, neck or limb stiffness; trismus (lockjaw); or sardonic smile (dystonia, or involuntary muscle contractions, producing a fixed smile). As the disease progresses, painful muscle spasms occur, affecting any muscles simultaneously. When spasms and rigidity affect the respiratory or laryngeal muscles, sedation and/or prolonged ventilation in the intensive care setting is required. Examples of complications of tetanus include hypertension (high blood pressure), hypotension (low blood pressure), respiratory failure, cardiac arrythmias (irregular heartbeat), pneumonia, fractures, muscle rupture, deep vein thrombophlebitis, pulmonary emboli, decubitus ulcers and rhabdomyolysis. In severe cases, tetanus can be fatal.
How is it transmitted?
Tetanus spores are found in dirt, dust and animal faeces. They can survive in the environment for many years. Spores can be introduced into the body via a wound where the bacteria can grow and cause infection. Spores can survive in a wound for as long as 3 months before becoming active.
Tetanus can occur following a significant wound or after trivial or even unnoticed wounds. Examples of wounds that are considered tetanus-prone include:
- bites, either animal or human
- deep penetrating wounds
- minor lacerations, for example, from cat scratches, rose thorns, glass or other foreign bodies (e.g. splinters)
- contusions or burns
- compound (open) fractures
- cuts or lacerations with outdoor equipment
- tooth reimplantation post avulsion (knocked out tooth)
- IV drug use sites.
Tetanus cannot be passed directly from person to person.
Epidemiology
The incidence of tetanus is rare in Australia. Since 2001, there have been fewer than 8 cases reported each year, with a total of 84 cases since 2000. The case fatality rate in Australia is about 2%. Globally, tetanus is fatal in approximately 11% of reported cases.
Tetanus can affect people of any age. However infections in Australia are more commonly seen in older adults who have never been vaccinated or were vaccinated more than 10 years ago.
In countries with low rates of vaccination, Maternal and Neonatal Tetanus (MNT) remains a common life-threatening consequence of unhygienic deliveries and umbilical cord care practices. Mortality rates associated with MNT are extremely high, especially when appropriate medical care is not available, as is often the case.
Prevention
Vaccination is the most effective way to prevent tetanus. Vaccines target the tetanus toxin (toxoid) and, in Australia, are available only in combination with diphtheria. Vaccines may also include other antigens, such as pertussis, inactivated poliovirus, hepatitis B and haemophilus influenzae type B.
Table: Which tetanus-toxoid containing vaccine to use by age of patient
| Age Group | Vaccine brand, antigen, dose and route | |||||
| Infanrix Hexa/Vaxelis (DTpa, hep B, Hib, polio) 0.5 mL IM | Infanrix/Tripacel (DTPa) 0.5 mL IM | Infanrix-IPV/Quadracel (DTPa, polio) 0.5 mL IM | Boostrix/Adacel (dTpa) 0.5 mL IM | Boostrix IPV/Adacel Polio (dTpa, polio) 0.5 mL IM | ADT (dT) 0.5 mL IM | |
| 6 weeks to < 10 years | ✓ | ✓ | ✓ | |||
| ≥ 10 years | * | ✓ | ✓ | ✓ |
Tick = recommended for use in this age group
Grey shading = not routinely recommended for use in this age group
* ATAGI recommends the use of Infanrix hexa and Vaxelis in children aged < 10 years, however the Royal Children’s Hospital (RCH) preferentially uses them up to < 18 years in instances where multiple vaccines are required (eg. catch up, post chemotherapy/post HSCT).
Primary course
As per the National Immunisation Program (NIP), a primary course of tetanus vaccination is given at 6 weeks, 4 months, and 6 months of age (Infanrix hexa/Vaxelis).
Boosters
Booster doses are scheduled to be administered at:
- 18 months (Infanrix/Tripacel)
- 4 years (Infanrix-IPV/Quadracel)
- 12 to 13 years of age/year 7 high school program (Boostrix)
Regular booster doses every 10 years are no longer routinely recommended. However, women who receive the recommended pertussis vaccine during every pregnancy will also receive a booster of tetanus (and diphtheria). Adults aged ≥ 50 years who have not received a dose of tetanus-containing vaccine in the last 10 years should consider a booster dose (unfunded).
Those who have a tetanus-prone wound should also receive a booster if they have not received a dose of tetanus-containing vaccine in the last 10 years (see Tetanus-prone wounds below).
Catch up
Any person with an incomplete tetanus immunisation history should be offered catch up. Refer to MVEC: Catch up immunisation for specific guidance.
Tetanus-prone wounds
The correct management of a tetanus-prone wound is vital for the prevention of tetanus infection.
Initial treatment involves cleaning and disinfecting the wound. Medical review by a GP or emergency department is recommended.
Reviewing a person’s immunisation history will inform tetanus-prevention management, which may include vaccination and/or the administration of immunoglobulin. Immunoglobulin provides short-term protection against tetanus. Vaccines provide longer and ongoing protection against the development of disease.
Regardless of the nature of the wound, all unimmunised individuals, partially immunised individuals(those who have received less than 3 doses of a tetanus-containing vaccine) and immunocompromised individuals are at the greatest risk of contracting tetanus.
Groups who are often assumed to be up to date, but may lack optimal vaccine protection, include:
- those aged between 9-13 years, as they are 5 + years post their 4-year-old vaccine and may not have yet received their Year 7 booster
- the elderly population, due to waning immunity and time passed since last booster.
Table: Guide to tetanus-prevention management
| Vaccine history of tetanus-containing vaccine | Time since last dose | Clean, minor wounds | Other wounds | ||
| Vaccination ¥ | Immunoglobulin | Vaccination ¥ | Immunoglobulin | ||
| ≥ 3 doses | < 5 years | * | |||
| 5–10 years | ✓ | * | |||
| > 10 years | ✓ | ✓ | * | ||
| < 3 doses / uncertain | Any time / uncertain | ✓^ | ✓^ | ✓ |
Tick = required
Shaded boxes = not required
¥ Using age-appropriate tetanus-containing vaccine (see above Table: Which tetanus-toxoid containing vaccine to use by age of patient)
*administration of immunoglobulin is recommended when the patient has humoral immune deficiency or HIV (regardless of CD4+ count)
^ where there is no documented history of a completed 3-dose primary course of tetanus vaccination patients should receive all missing doses AND tetanus immunoglobulin.
Vaccine side effects
Injection site reaction (ISR) is a common side effect after receiving a tetanus-toxoid vaccine. Uncommon side effects include headache, lethargy, irritability, body aches and fever. These side effects are usually self-limiting and resolve within 48 hours.
Resources
- Australian Immunisation Handbook: Tetanus
- The Royal Children’s Hospital Clinical Practice Guideline: Management of tetanus prone wound
- Australian Government Department of Health and Aged Care: Changes to the National Immunisation Program schedule from July 1 2023
Author: Lynne Addlem (Immunisation Nurse, The Royal Children’s Hospital) and Rachael McGuire (Education Nurse Coordinator)
Reviewed by: Katie Butler (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: November 2023
Materials in this section are updated as new information becomes available. The Melbourne Vaccine Education Centre (MVEC) staff regularly review materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Travel medicine
Travel medicine focuses on disease prevention and injury risk minimisation with advice based upon the travelling person’s individual circumstances such as the location and purpose of travel, duration and time of year, as well as any underlying medical conditions or other risk factors.
Where to get travel advice
General Practitioners and travel medicine clinics can provide recommendations for preventative care and offer vaccination. There are also paediatricians who specialise in infectious diseases or immunisation who can provide advice for children (and their families). There are also a number of excellent websites that can be accessed to obtain country specific updates and information on emerging infections [see Resources below].
When to seek advice
For specific pre-travel advice, consultation with a health care professional is best done a minimum of 4-6 weeks prior to departure, which allows time to develop immunity following any recommended vaccines prior to arrival at the travel destination. Depending on which vaccines are recommended, a course of vaccines over time may be indicated before a person is considered fully protected.
As most travel specialists require a GP referral and may have wait times for an appointment, it is important to factor this into travel planning timelines.
Vaccines
Some routine vaccines can be given earlier than scheduled or as additional doses for the purposes of travel. Examples of this include:
- MMR (measles, mumps and rubella) vaccine: an extra, funded dose can be administered between the age of 6-11 months if travelling to an area where measles are endemic
- Meningococcal vaccines:
- MenACWY (Nimenrix) vaccines can be administered from 6 weeks of age in order to provide earlier protection (scheduled on the NIP at 12 months of age and in year 10 of secondary school)
- Meningococcal B (Bexsero) vaccines can also be given from 6 weeks of age (not funded on the National Immunisation Program (NIP) for all, but can be purchased privately for those who do not qualify for a funded dose)
- Influenza vaccine: depending on the time of year and destination of travel, it may be advisable to receive an influenza vaccine
- COVID-19 vaccines: due to ongoing emerging strains of COVID-19 disease, it is important to ensure a primary course of COVID-19 vaccination is complete along with any recommended boosters based on current recommendations.
Other travel-related vaccines, depending on your location and duration of travel might include:
- BCG
- Cholera
- Hepatitis A
- Japanese encephalitis
- Typhoid
- Rabies
- Yellow fever
Other precautions
Other preparations for travel may include:
- considering the need for malaria prophylaxis (medication to prevent malaria)
- measures to prevent mosquito-borne disease such as wearing long-sleeved clothing, mosquito nets, and insect repellent than contains DEET
- food and water safety.
Immunocompromised and pregnant travellers
Individuals who are immunocompromised as a result of a medical condition or specific therapies are encouraged to seek travel advice early to maximise protection against vaccine preventable diseases prior to travel.
Similarly, women who are planning pregnancy or who are pregnant, should seek travel advice as early as possible to ensure optimum protection for both the mother and unborn baby. Women planning pregnancy with a high likelihood of travel, should consider vaccination prior to pregnancy noting that live-attenuated vaccines are contraindicated during pregnancy.
Visiting friends and relatives (VFR)
VFR travel is one of the most common reasons for international travel. A VFR traveller is an immigrant who travels from the high-income country they are living in to their lower-income country of birth to visit friends or relatives. A travel consult prior to VFR travel should consider whether an individual has had routine NIP vaccines or a history of vaccine preventable disease/s as well as vaccines indicated for the country they are travelling to. Rates of some travel-related infections are higher in this population and taking appropriate precautions such as vaccination and considering food and water safety is important in ensuring optimum protection.
Resources
MVEC travel related resources
Other resources
- Department of Health Victoria: Yellow fever vaccination centres
- The RCH kids info- travel health advice
- Centres for Disease Control and Prevention (CDC): Travelers’ health
- Bug off
- Travel health pro- travel health website
Authors: Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael Purcell (RCH Immunisation Fellow), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: June 8 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.