Immunosuppression In Pregnancy and Infant Vaccine Recommendations
Background
Immunosuppressive therapies play an important role in the treatment of many medical conditions. Biologic disease-modifying anti-rheumatic drugs (bDMARDs) are a specific subset of immunosuppressive medications primarily used for the treatment of inflammatory rheumatic diseases. However, they are increasingly being used to treat many other conditions. Examples of bDMARDs include adalimumab, tocilizumab, etanercept, infliximab and rituximab.
If exposed to vaccine-preventable diseases, individuals with suppressed immune systems may have an increased risk of developing severe disease (and of hospitalisation, intensive care admission and death). However, some vaccines (live-attenuated vaccines) may be contraindicated in individuals with immunosuppression due to the potential risk of vaccine-related disease. Additionally, immune responses to vaccines may be suboptimal.
Immunosuppression use in pregnancy and infant vaccines
Due to the broadened scope of use of bDMARDs, including during pregnancy, there is an increasing number of infants exposed to bDMARDs in utero. The effect that this has on an infant’s immune system is not well understood, but there is the potential for significant implications regarding the safe use and effectiveness of routine and additional vaccines in infants.
Recommendations
The following guidance outlines recommendations for specific investigations and vaccines for infants exposed to maternal immunosuppression (bDMARDs) in utero. This guidance has been developed as a collaboration between MVEC, Queensland Children’s Hospital and Royal Brisbane and Women’s Hospital.
Vaccination recommendations for infants exposed to maternal immunosuppression (PDF)
NB: This document provides guidance on the maternal use of bDMARDs only (not other conventional DMARDs). It outlines the implications for infants and does not replace investigations or vaccine recommendations for pregnant women who are receiving/have received bDMARDs.
Resources
- Guidance: Vaccination recommendations for infants exposed to maternal immunosuppression (PDF)
- MVEC: Immunosuppression and vaccines
- MVEC: Rituximab and immunisation recommendations
- The Society of Hospital Pharmacists in Australia: bDAMRDS quick reference guide
Authors: Angela Berkhout (Paediatric Infectious Diseases Physician & General Paediatrician, Children’s Health Queensland), Sophie Wen (Paediatric Infection Specialist, Children’s Health Queensland) , Michael Nissen (Infectious Diseases, Microbiology and Paediatric Consultant, Royal Brisbane and Women’s Hospital), Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director, SAEFVIC and MVEC, Murdoch Children’s Research Institute)
Date: December 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
International Network of Special Immunization Services
Background
The International Network of Special Immunization Services (INSIS) is a coordinated international collaboration of vaccine safety surveillance systems. By collaborating on a global scale, rare serious adverse events following immunisation (occurring in < 1 per 10,000 vaccine recipients) are more likely to be identified, thoroughly investigated and better understood. The international leads are Karina Top (Canada) and Bob Chen (United States).
Objectives
INSIS aims to promote confidence in the safety of vaccines. Implementing standardised case definitions and protocols can assist with identifying unique molecular signatures and biomarkers associated with rare adverse events following immunisation (AEFI) and improve our understanding of the genetic basis for adverse events (eg. thrombosis with thrombocytopenia syndrome and myocarditis/pericarditis following COVID-19 vaccines). By determining the causes of AEFI and identifying individual risk factors for experiencing AEFIs, it can develop recommendations for the safest way to immunise individuals with a history of or those identified as at higher risk of experiencing an AEFI. This improved understanding will also help in vaccine safety communication and the development of resources through leading websites such as MVEC.
SAEFVIC, in collaboration with AEFI-CAN, leads Australia’s involvement with INSIS.
Funding
INSIS is funded by the Coalition for Epidemic Preparedness Innovations (CEPI), the Canadian Institutes of Health Research (CIHR) and IWK Health Centre.
Resources
- International Network of Special Immunization Services
- MVEC: SAEFVIC
- MVEC: AusVaxSafety
- MVEC: AEFI-CAN
- MVEC: Adverse event reporting Australia
Authors: Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director, SAEFVIC, Murdoch Children’s Research Institute)
Date: March 9, 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Immune thrombocytopenia (ITP)
Background
Immune thrombocytopenia, also previously known as idiopathic thrombocytopenia purpura (ITP), is an uncommon autoimmune condition in which the body’s own immune system attacks platelets (the cells found in the blood which normally help the blood to clot). In ITP, the body produces antibodies which attack and destroy platelets, which decreases their number and can cause symptoms such as bruising, pin-point red spots (petechiae) on the skin or bleeding (e.g. nose bleeds or bleeding from the gums). Some people have no symptoms at all.
ITP is most often triggered by a viral illness, occurring in the weeks prior to symptoms developing. ITP can be acute (lasting less than 6 months) or chronic (lasting longer than 6 months), with acute ITP far more common in children and chronic ITP far more common in adults. The symptoms of acute and chronic ITP are the same. Approximately one in every 10,000 child will be affected by ITP.
Some cases of ITP are discovered by chance. In some instances, ITP may go away without any treatment. However, when platelets are very low or there are symptoms of bleeding treatment may be needed. Corticosteroids and intravenous immunoglobulin are the most common forms of initial treatment.
ITP and measles, mumps and rubella vaccines (MMR)
MMR vaccines have been associated with ITP, with the risk estimated at approximately 1 in 25,000 vaccinations. However, the risk of ITP following MMR vaccine is much lower than the risk seen with natural measles or rubella infections. Patients with a history of ITP are still recommended to receive the MMR vaccine in line with the National Immunisation Program (NIP) as although there is a small risk of relapse, this risk is still present with the viruses themselves, and it is important that people are protected against these viruses which can cause significant morbidity and mortality.
ITP and COVID-19 vaccines
A link between COVID-19 vaccines and ITP is currently being investigated. This is because of the known link between MMR vaccine and ITP, and also because ITP can be triggered by COVID-19 infection. A study in Scotland reported a link between Vaxzevria (AstraZeneca) and ITP, with rates seen post vaccination higher than background rates of ITP in the community . To date, there has been no association found between mRNA COVID-19 vaccines (Comirnaty or Spikevax) and ITP. Monitoring and investigations are and underway in Australia.
Should patients with a history of ITP receive COVID-19 vaccines?
Yes. The effect of COVID-19 vaccination on pre-existing ITP (acute and chronic) has not been well characterised. Limited and early data indicates that vaccination may worsen thrombocytopenia in approximately 10% of patients with chronic ITP post vaccination. However, it is important to note that ITP is most often triggered by a virus, and that the risk of relapse or worsening of ITP is likely higher if these patients contract COVID-19 than the risk following vaccination itself. Patients with a history of ITP are therefore recommended to proceed with vaccination, however, if clinical symptoms worsen post vaccination (days to weeks) then monitoring of platelets and escalation of therapy may be required.
Can patients who develop ITP following a dose of COVID-19 vaccine receive future doses?
Yes. Patients who develop ITP following receipt of a COVID-19 vaccine can receive future doses (including booster doses) once they are advised that it is safe to do so. Vaccination should be deferred until platelets are stable (>50 x 109/L and off any ITP treatment for >3 months). If a patient remains on immunosuppression (eg. corticosteroids) they should discuss with a heamatologist whether to proceed.
If a patient (> 18 years) has ITP following a dose of Vaxzevria (AstraZeneca), they should receive an mRNA vaccine (eg. Comirnaty or Spikevax) or Nuvaxovid (Novavax) for their subsequent doses. If a patient has ITP following the first dose of an mRNA vaccine or Nuvaxovid they should proceed with the second dose of the same vaccine, as no association has been found between these vaccines and ITP to date. There remains a risk of relapse regardless of vaccine brand, however this risk of relapse is higher with COVID-19 disease itself. Close monitoring should occur following vaccination with dose 2 to ensure that there is no further drop in platelets.
ITP and other vaccines
There are some small studies or case reports which suggest a possible increased risk of ITP and other vaccines, such as influenza, HPV, polio and pneumococcal vaccines. However, to date, no vaccines have been proven to be associated with ITP other than the MMR and Vaxzevria (AstraZeneca) vaccines described above. People with a history of ITP are safe to receive all routine NIP and travel vaccines as required.
Resources
- Miller E, Waight P, Farrington CP, Andrews N, Stowe J, Taylor B. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84(3):227-9
- Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107-11
- Bhattacharjee S, Banerjee M. Immune Thrombocytopenia Secondary to COVID-19: a Systematic Review. SN Compr Clin Med. 2020:1-11
- Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290-7
- Rinaldi M, Perricone C, Ortega-Hernandez OD, Perricone R, Shoenfeld Y. Immune thrombocytopaenic purpura: an autoimmune cross-link between infections and vaccines. Lupus. 2014;23(6):554-67
- Garbe E, Andersohn F, Bronder E, Salama A, Klimpel A, Thomae M, et al. Drug-induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68(5):821-32
Authors: Sally Gordon (VicSIS Manager, Department of Health), Paul Monagle (Clinical Haematologist, Royal Children’s Hospital), Francesca Machingaifa (MVEC Education Nurse Coordinator), Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Paul Monagle (Clinical Haematologist, Royal Children’s Hospital), Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: July 21, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Influenza
What is it?
Influenza viruses are single stranded RNA orthomyxoviruses which can cause acute viral infections of the respiratory tract. Infections are often classified according to the type of influenza virus responsible for the infection, typically A, B, or C. Influenza types A and B are more commonly responsible for causing severe disease. Influenza A can be further subtyped based on the differences in surface antigens.
What to look for
The incubation period of influenza disease is 1-4 days with typical symptoms including fever, headache, myalgia (muscle aches), lethargy (tiredness), coryza (runny nose), sore throat and cough. Gastrointestinal symptoms such as nausea, vomiting and diarrhoea can also occur. Children with influenza will often present with symptoms of croup.
Most influenza infections will resolve within 2-7 days. However, complications including otitis media (ear infections), secondary bacterial pneumonia (lung infections) and encephalitis (brain inflammation) can prolong the illness and disease outcomes.
How is it transmitted
Influenza is highly contagious. Transmission is via respiratory droplets, aerosol or through direct contact with the respiratory secretions of an infected person.
Epidemiology
Influenza disease can occur as sporadic cases, as an epidemic, or as a pandemic. Whilst outbreaks more commonly occur in the winter months in temperate climates, there is a greater variation seen in the timing of cases in the tropics.
Aged care facilities, health care facilities and child care centres are well recognised as high-risk areas for influenza outbreaks.
Pregnant people, children < 5 years of age, those aged > 65 years, people with underlying medical conditions, and Aboriginal and Torres Strait Islander peoples carry the highest rates of morbidity and mortality within Australia.
Prevention
Influenza vaccination is available and recommended for anyone ≥ 6 months of age who wishes to be protected against influenza disease and its complications. Due to the circulating strains of influenza virus changing each year, vaccination is recommended annually to provide the most effective protection against disease.
Influenza vaccines are provided for free on the National Immunisation Program (NIP) in 2024 for high-risk groups, including:
- children aged 6 months to < 5 years
- all adults ≥ 65 years of age
- specific populations aged 5 years to 64 years who are at a greater risk of developing complications from influenza (including pregnant people, First Nations people, and those with certain medical risk factors).
Those aged 5 to 64 years who do not qualify for funded vaccines can purchase vaccines privately through some councils, GPs and pharmacies.
Vaccine platform
The influenza vaccines available in Australia are inactivated, meaning that they cannot replicate and cause influenza disease. They can be cell-based or egg-based depending on how they are manufactured.
Traditional influenza vaccines are made by cultivating influenza viruses in chicken eggs. Cell-based influenza vaccines are made by growing influenza viruses in animal cells lines (canine kidney). ATAGI has no preference for use of cell-based influenza vaccines over traditional egg-based vaccines (individuals with egg allergy/anaphylaxis can safely receive egg-based influenza vaccines, see commonly asked questions below).
Table 1: The influenza virus strains included in the 2024 seasonal influenza vaccines
| Egg-based influenza vaccines | Cell-based influenza vaccines |
| A/Victoria/4897/2022 (H1N1)pdm09-like virus | A/Wisconsin/67/2022 (H1N1)pdm09-like virus |
| A/Thailand/8/2022 (H3N2)-like virus | A/Massachusetts/18/2022 (H3N2)-like virus |
| B/Austria/1359417/2021-like (B/Victoria lineage)-like virus | B/Austria/1359417/2021 (B/Victoria lineage)-like virus |
| B/Phuket/3073/2013-like (B/Yamagata lineage)-like virus | B/Phuket/3073/2013 (B/Yamagata lineage)-like virus |
Whilst the World Health Organization (WHO) and the Australian Influenza Vaccine Committee (AIVC) recognise that the B Yamagata strain has not been circulating for several years and its inclusion in annual vaccines is no longer warranted, it has been included in the 2024 influenza vaccines. This is not a safety concern and WHO and the AIVC support its inclusion (refer to resources below).
Adjuvanted and high-dose vaccines
Due to a gradual decline in effectiveness of the immune system of older people (a process known as immunosenescence) immunity following vaccination with standard influenza vaccines can be suboptimal. In addition, those aged ≥ 65 years have the highest rates of influenza disease burden and associated complications including pneumonia and death. Therefore, to increase the immune response adjuvanted (Fluad Quad) or high-dose (Fluzone High-dose Quad) influenza vaccines are the preferred vaccine type for the older population.
Table 2: The 2024 influenza vaccine brand recommendations according to age
| Age Group | Vaccine brand and dose | |||||||
| Vaxigrip Tetra (0.5 ml) | Fluarix Tetra (0.5 ml) | Afluria Quad (0.5 ml) | FluQuadri (0.5ml) | Influvac Tetra (0.5ml) | Flucelvax Quad (0.5ml) | Fluad Quad (0.5ml) | Fluzone High-Dose Quad (0.7ml) | |
| < 6 months | ||||||||
| 6 months - < 5 years | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ✓*^ | |||
| ≥ 5 - < 60 years | ✓*Ω^ | ✓*Ω^ | ✓*^ | ✓*^ | ✓*^ | ✓*Ω^ | ||
| ≥ 60 - < 65 years | ✓Ω^ | ✓Ω^ | ✓^ | ✓^ | ✓^ | ✓Ω^ | ✓# | |
| ≥ 65 years | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓# | ✓# |
* 2 doses, minimum of 4-weeks apart should be given to children < 9 years of age in the first year of receiving the influenza vaccine, a single dose is recommended in subsequent years.
ΩNIP funding only for First Nations people, pregnant people and people with certain medical risk factors
#Adjuvanted or high-dose quadrivalent influenza vaccines are preferentially recommended for people adults ≥ 65 years.
βFluarix-tetra/FluQuadri/Afluria Quad/Vaxigrip tetra/Influvac tetra/ Flucelvax Quad are registered for use in people aged ≥ 65 years, however adjuvanted or high-dose vaccines are the preferred vaccines for this age group.
^2 doses are recommended in the first year following solid organ transplant (SOT) or haematopoietic stem cell transplant (HSCT) regardless of history of influenza vaccination due to immunosuppression. The exception to this is in individuals receiving an adjuvanted or high-dose influenza vaccine where only 1 dose is recommended.
shaded boxes indicate vaccines funded under the NIP for eligible individuals.
shaded boxes not registered for use in this age group.
shaded boxes indicate adjuvanted or high-dose vaccines.
Expected side effects
Common side effects following vaccination include pain, redness and swelling at the injection site as well as fever, malaise and myalgia. Symptoms usually occur within the first 24-48 hours following immunisation.
Cell-based influenza vaccines have a similar side-effect profile to traditional egg-based influenza vaccines. Side effects may occur slightly more commonly following immunisation with adjuvanted quadrivalent formulations than with standard influenza vaccine formulations.
Commonly asked questions
When is the ideal time to be immunised against the flu?
Annual vaccination before the onset of influenza season is recommended for all individuals ≥ 6 months of age. The peak period of circulating influenza disease in Australia is typically June to September. However, out of season cases can and do occur. Optimal protection against influenza occurs within the first 3-4 months following vaccination. It is never too late in the season to vaccinate.
Pregnant women can safely receive the influenza vaccine during any stage of pregnancy. Where a pregnancy crosses over seasons, some pregnant women may be recommended to receive 2 influenza vaccines, one from each year.
Do healthy people need to be immunised against influenza?
Influenza can be a very serious disease resulting in hospitalisation and death. Even in cases where disease and its complications are not severe, it can cause a great inconvenience for the individual, including the cost of GP visits and medications, as well as time off work for themselves or to care for their sick child.
In some cases, a person may not get severe disease but infection can be spread to other people. This can be significant when it is spread to those who are too young to be immunised or are at higher risk of complications of disease.
If an individual has had confirmed influenza disease this year, are they still recommended to receive an influenza vaccine and when should they receive it?
Vaccination is still recommended for someone with a history of confirmed influenza infection as the vaccine protects against multiple strains of influenza disease. The influenza vaccine can be administered as soon as the patient has recovered from their illness.
Can the influenza vaccine be given at the same time as other vaccines?
Yes, influenza vaccines may be co-administered with any other vaccine on the same day. This includes live-attenuated vaccines (e.g. measles and varicella) and the pertussis vaccine in pregnancy.
If a patient received a 2023 influenza vaccine at the end of the season in early 2024, do they still need a 2024 influenza vaccine?
Yes. A 2024 influenza vaccine is still recommended in order to provide protection against this year’s circulating strains. A minimum interval of 4 weeks is recommended.
In children < 9 years of age who received the influenza vaccine for the first time last year but only received 1 dose, how many doses are required this year?
Only 1 dose is required in this instance. 2 doses of the influenza vaccine are recommended for children < 9 years of age in the first year of receiving the vaccine. However, if the second dose was inadvertently missed, it does not require catch up and only 1 dose is required in future years.
Are influenza vaccines safe for people with allergies?
Based on prospective and retrospective studies of influenza vaccination in those with and without egg allergy (including egg anaphylaxis), the presence of egg allergy does not increase the risk of allergic reactions to the influenza vaccine. Egg-based influenza vaccines can be administered in community vaccination clinics (which may or may not have direct medical practitioner supervision), General Practitioner surgeries or immunisation clinics as a single dose followed by the recommended 15 minute observation period. It is not necessary to preferentially administer cell-based influenza vaccines in this patient group.
All influenza vaccines available under the NIP in 2024 are latex free meaning that people with a latex allergy can safely be vaccinated.
For further queries on influenza vaccination, please contact us via our immunisation support.
Resources
- ATAGI statement on the administration of seasonal influenza vaccines in 2024
- TGA: AIVC Recommendations for the Composition of Influenza Vaccines for Australia in 2024
- Department of Health: Seasonal influenza vaccine
- Department Health: 2024 influenza vaccination – Program advice for health professionals
- Australian Immunisation Handbook: Influenza
- Australasian Society of Clinical Immunology and Allergy: Vaccination of the egg allergic individual
- MVEC: Maternal vaccination during pregnancy
Authors: A/Prof Nigel Crawford (Director SAEFVIC, Murdoch Children’s Research Institute), Rachael McGuire (Research Nurse SAEFVIC, Murdoch Children’s Research Institute), Georgina Lewis (Clinical Manager SAEFVIC, Murdoch Children’s Research Institute) and Mel Addison (Research Nurse SAEFVIC, Murdoch Children’s Research Institute).
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator).
Date: March 2024
Materials in this section are updated as new information becomes available. The Melbourne Vaccine Education Centre (MVEC) staff regularly review materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Immunisation recommendations for the older population
There are a variety of factors that need to be considered in relation to the vaccination of the older population. A gradual decline of the immune system occurs as people age (known as immunosenescence), impacting how the immune system responds to new infections, as well as the effectiveness of long-term immune memory. It is for this reason that some vaccines are specifically designed for the older population and aim to enhance the immune response by using higher immunogenicity formulations or by containing adjuvants. Providing optimal protection can also be complicated due to the increasing prevalence of multiple co-morbidities in the ageing population. Specific medical conditions or targeted therapies (eg. chronic renal conditions, chemotherapy for cancers etc) can also cause older adults to be more vulnerable to infections and their complications. Further to this, relying on patient recall, as well as a lack of awareness for the recommended vaccines for the older population, can result in either missed vaccines or additional unnecessary doses being administered.
There are multiple vaccines recommended for the older population as outlined below.
Herpes zoster (Shingles) vaccines
Shingles is caused by a reactivation of the varicella virus and will occur in approximately 20-30% of people in their lifetime. Older people (> 70 years of age) are more likely to suffer post-herpetic neuralgia (PHN) following a shingles infection than younger people. PHN is a chronic neuropathic pain which can affect 1 in 4 cases of shingles diagnosed in those > 80 years. It can persist for months to years with pain control being difficult to manage, impacting quality of life.
There are currently 2 vaccines available in Australia for the prevention of shingles:
- Zostavax®- a live-attenuated vaccine
- Shingrix®- an adjuvanted recombinant varicella zoster virus glycoprotein E (gE) subunit (non-live) vaccine
Zostavax®
Zostavax® has been shown to reduce the incidence of developing shingles by up to 50%, as well as the incidence of PHN in those ≥ 60 years of age by 66%. It is currently funded under the National Immunisation Program (NIP) for persons aged 70 years, with a catch-up program for those aged 71–79 years also funded (until October 2023). As it is a live-attenuated vaccine, it is contraindicated for use in those who are immunosuppressed, or on immunosuppressive medications (eg; Rituximab, Azathioprine, Prednisolone, chemotherapy etc). Prior to administering Zostavax® it is important to take a thorough patient history to determine suitability for immunisation.
Shingrix®
Shingrix® is preferred over Zostavax® for the prevention of shingles due to a higher efficacy, particularly in the older population. In those aged ≥ 50 years, Shingrix® provided 97% protection against shingles in immunocompetent individuals and 91% protection in those aged > 70 years. Clinical trials have demonstrated high efficacy up to 4 years following vaccination with immunogenicity data indicating this is likely to persist beyond 10 years.
Shingrix® is registered for use in adults aged ≥ 50 years. It is only available through private prescription and supplies are currently limited. It is a non-live vaccine and as such can safely be administered to immunocompromised individuals. ATAGI recommends a 7 day interval between the administration of COVID-19 vaccines and Shingrix®, and prefers that FluadQuad and Shingrix® are not co-administered on the same day.
Further guidance can be provided by reviewing the Australian Immunisation Handbook: Table. Live shingles vaccine (Zostavax) screening for contraindications or by contacting SAEFVIC prior to immunisation.
Pneumococcal vaccines
Invasive pneumococcal disease (IPD) can manifest as meningitis, pneumonia and bacteremia, with severe disease requiring hospitalisation, causing significant morbidity and even death. The elderly (along with infants) are at the highest risk of developing IPD. Recommendations for pneumococcal vaccines in adults vary according to age and medical condition [refer to ATAGI clinical advice on vaccination recommendations for people with risk conditions from 1 July 2020]. Pneumococcal vaccines are currently provided for free on the NIP for the following people:
- Aboriginal and Torres Strait Islander adults with NO risk condition – 1 dose of Prevenar 13® at 50 years of age, followed 8 weeks later by 2 doses of Pneumovax® 23, given 5 years apart
- Non-indigenous adults with NO risk condition – 1 dose of Prevenar 13® at >70 years
- Non-indigenous adolescents/adults diagnosed with a risk condition – 1 dose of Prevenar 13® at diagnosis, followed by 2 doses of Pneumovax® 23, given 5 years apart
In adults, injection site reactions may occur > 3 days following the Prevenar 13® dose given at > 70 years, particularly in those who have previously received Pneumovax®23. A history of large local injection site reactions following previous pneumococcal vaccines is not a contraindication to further doses.
Refer to MVEC: Pneumococcal for more information.
Influenza vaccines
For older adults, and those with certain medical conditions (eg. chronic lung disease, cardiac disease, immunosuppression), influenza disease can cause serious morbidity and mortality. Annual influenza vaccination is strongly encouraged and it is available for free on the NIP for those ≥ 65 years of age and/or adults with certain medical conditions. Due to a reduced immune response to routine influenza vaccines, those aged ≥ 65 years should receive higher-immunogenicity influenza vaccines.
Refer to MVEC: Influenza for specific information on brands and dosing.
COVID-19 vaccines
Older people and those with comorbidities (eg. hypertension, diabetes, chronic lung disease etc) are much more likely to suffer from severe COVID-19 disease if infected. Of those who are > 80 years of age and have COVID-19 disease, approximately 1 in 3 will die from it.
COVID-19 vaccination requires a 2-dose primary course for immunocompetent individuals, or a 3 -dose primary course for those with immunocompromise. A primary course shoulod be followed by a booster dose ≥ 3 months later, and a further “winter booster” dose ≥ 3 months after that for select individuals.
For more information on COVID-19 vaccination for older people please refer to COVID-19 vaccination – COVID-19 vaccination decision guide for frail older people, including those in residential aged care facilities.
Considerations for residents of residential aged care facilities (RACF)
Whilst every effort should be made to immunise residents of RACF at risk of vaccine preventable diseases like COVID-19 and influenza, it is important to monitor for adverse events following immunisation (AEFIs). Due to a high incidence of cognitive impairment, elderly residents may not have capacity to self-report any side effects. Any AEFIs experienced within 5 days of vaccination should be reported to SAEFVIC. It is important to monitor for non-specific symptoms seen in the elderly population when unwell such as falls, delirium, functional decline, decrease/loss of appetite or changes in mood/behaviour.
For further information on additional cares that may be required and management of symptoms following vaccination in residents of RACF refer to Guidance for vaccination care of residents of Victorian Residential Aged Care Facilities.
Reporting to the Australian Immunisation Register (AIR)
The AIR provides a record of all vaccine doses given, the date of administration as well as the specific brands used. Since 2016 vaccines administered to Australians of any age have been recorded onto the AIR. Patient recall, particularly in the older population, is not reliable and as such it important that immunisation records are accurately maintained and reviewed regularly.
From March 2021, new legislation came into effect making reporting vaccines to AIR mandatory. This includes all COVID-19 vaccines, influenza vaccines and all National Immunisation Program vaccines.
Resources
- Australian Government Department of Health: Immunisation for Seniors
- MVEC: Influenza
- MVEC: Zoster
- MVEC: Pneumococcal disease and vaccines
- MVEC: COVID-19 vaccines: Frequently asked questions
- MVEC: Australian Immunisation Register
- COVID-19 vaccination – COVID-19 vaccination decision guide for frail older people, including those in residential aged care facilities
- Guidance for vaccination care of residents of Victorian Residential Aged Care Facilities
- Aw D. Silva B. and Palmer D. Immunosenescence: emerging challenges for an ageing population Immunology 2007 Apr; 120(4): 435–446
- Gustafson C. Kim C. Weyand C. Goronzy J Influence of immune aging on vaccine responses Journal of Allergy and Clinical Immunology 2020 May; 145(5): 1309–1321
Authors: Daryl Cheng (Paediatrician, The Royal Children’s Hospital), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed by: Francesca Machingaifa (MVEC Education Nurse Coordinator)
Date: August 5, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Identifying AEFI in diverse skin colour
Background
The assessment of skin for clinical signs and symptoms is important when identifying adverse events following immunisation (AEFI). Most dermatological assessment guidelines commonly refer to the presentation of symptoms in patients with light skin tones.
The skin is one of the most complex organs in the body. It consists of 3 layers, with each layer performing different roles. The colour of a person’s skin is influenced by the amount of melanin (natural pigment) which is produced by the melanocytes located in the epidermis (outer layer of the skin), with darker toned skin having more melanin present than lighter toned skin. The amount of melanin will affect the appearance of AEFI on a patient’s skin and therefore AEFI, such as pallor, cyanosis, erythema and urticaria, may appear differently in varied skin tones. This can pose a challenge for immunisation providers to identify symptoms of AEFI in a timely manner.
Pallor
Pallor refers to the pale appearance of the skin, nail beds and mucous membranes. Pallor is not always a symptom of disease.
Pallor may be difficult to detect in dark toned skin and may present as ashen or grey. In brown toned skin the skin will present more yellowish in colour. An alternative method for identifying pallor in darker skin tones can be assessing the palmer surface which can appear paler.
Following immunisation, pallor can be noted in events such as vasovagal syncope (fainting) and hypotonic hypo-responsive episodes (HHE).
Cyanosis
Cyanosis is a symptom of decreased oxygen in the bloodstream. There are 2 types of cyanosis:
- Peripheral cyanosis, which is observed in the extremities including hands, fingertips and feet.
- Central cyanosis, which is detected in central parts of the body including head, torso and mucous membranes and is often more serious.
In those with light skin tones, cyanosis will present as a bluish/purple hue. In patients with naturally yellow toned skin, cyanosis may cause a grayish-greenish appearance. In those with darker skin tones, cyanosis may be trickier to assess and may be observed as grey or white.
Following immunisation, cyanosis may occur in the setting of a HHE, apnoea, breath holding episode or high fever.
Erythema
Erythema describes a red appearance of skin caused by the dilation of superficial blood vessels and increased blood flow. It often occurs with skin trauma, inflammation, infection or rash.
Erythema is clearly visible on light toned skin. It may appear red or cause a purplish discoloration on some darker toned skins however it is difficult to see on very dark skin. Assessing the affected site or area for other signs including warmth, swelling or induration can assist in clinical assessment and diagnosis of erythema.
Following immunisation, erythema can be widespread or localised at the injection site.
Urticaria
Urticaria, or hives, occur when the mast cells that lie within the skin release histamine that irritates nerve endings and causes local blood vessels to dilate and leak fluid. They can appear anywhere on the body and can be transient in nature. The cause is often not known however, they may be associated with infections or allergy.
On light toned skin, hives appear as itchy, raised red welts. They often have a white center or wheal which looks like a mosquito bite and are surrounded by an erythematous ring. In darker toned skin, hives appear as raised lumps however the colour changes to the skin may not be as obvious.
Following immunisation, hives can occur anywhere on the body and may occur in the setting of an allergic reaction.
Resources
Images
Other resources
- DermNetNZ: Ethnic dermatology
- Australasian Society of Clinical Immunology and Allergy: Urticaria
- Ortonne, J. Normal and abnormal skin colour, Annales de Dermatologie et de Venereologie December 2021 (139):S125-S129
- Sommers, M. Color Awareness: A must for patient assessment, American Nurse, January 2011
- The Dermatologist: Identifying erythema in skin of colour
Authors: Georgina Lewis (Clinical Manager SAEFVIC, Murdoch Children’s Research Institute), Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Francesca Machingaifa (SAEFVIC Research Nurse, Murdoch Children’s Research Institute) and Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Rachael McGuire (MVEC Education Nurse Coordinator)
Date: May 31, 2022
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Injection site nodules
Background
The development of an injection site nodule (ISN) is a rare adverse event following immunisation (AEFI). Nodules can occur following administration of any injected vaccine. They usually present in the days or weeks following immunisation. ISNs are most often reported following vaccines given in infancy or childhood. An ISN usually persists for weeks or months. Very rarely, a nodule may persist for years. ISNs are most often asymptomatic, but may be intermittently tender, itchy, or show overlying skin changes (e.g. flaky skin). They generally resolve spontaneously, without treatment or investigation.
Diagnosis
ISNs are defined as a firm, discrete or well-demarcated soft-tissue lump at the site of vaccination, in the absence of heat, erythema (redness) or signs of abscess (e.g. pus, pain). They are often described by parents and caregivers as a pea-size lump under the skin.
Diagnosis is generally based on a healthcare provider’s clinical assessment. Although not routinely recommended, some clinicians may request an ultrasound of the area to confirm or support the diagnosis, and to exclude alternative diagnoses.
ISNs that last for months or years, with symptoms, are referred to as ‘persisting subcutaneous nodules’ (subcutaneous meaning under the skin). Itch is generally the concerning symptom that motivates people to seek medical attention. Ongoing scratching can alter the appearance of the skin leading to excoriation (irritated skin), hair growth and pigmentation changes.
Intensified itching and an increase in size of the nodule can occur during the course of a viral illness, or following subsequent vaccinations administered at a different anatomical site.
Association and incidence
It is unclear what causes an ISN. Factors identified as possibly contributing to ISNs include administration technique, vaccine components (including adjuvants, such as aluminium), patient predisposition and normal immune-mediated responses.
There is limited data on ISN incidence rates. Some estimates are available based on small, local cohorts.
The following references provide additional context on the possible causes and incidence of ISNs:
- Silcock R, Crawford NW, Selvaraj J, McMinn A, Danchin M, Lazzaro T, et al. Subcutaneous nodules following immunization in children; in Victoria, Australia from 2007 to 2016. Vaccine. 2020 Mar 5;38:3169–3177. doi:10.1016/j.vaccine.2019.12.066
- Silcock R, Crawford NW, Perrett KP. Subcutaneous nodules: an important adverse event following immunization. Expert Rev Vaccines. 2019 Mar 11;18(4);405-410. doi:10.1080/14760584.2019.1586540
- Bergfors E, Lundmark K, Nyström Kronander U. A child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines. BMJ Case Rep. 2013;2013:bcr2012007779. Published 2013 Jan 24. doi:10.1136/bcr-2012-007779
Treatment
A conservative approach to treatment is recommended; ISNs generally resolve on their own without intervention. On some occasions, health professionals might advise topical corticosteroids to treat the itch or skin changes, and dressings to protect the area from vigorous scratching. Rarely, excision of the nodule may be considered by a specialist, based on nature and severity of symptoms.
Any AEFI should be reported to the vaccine safety service in your state. In Victoria, reports can be made to SAEFVIC.
Implications for future doses
It is recommended that people who experience ISNs continue to receive future vaccines according to the immunisation schedule. The history of, or presence of, a nodule is not a contraindication to future vaccines.
Ensure correct vaccine administration for both intramuscular (IM) and subcutaneous (SC) vaccines. For IM vaccines, consider deep IM injection to minimise the risk of potential recurrence of a nodule. Where possible, avoid vaccination at a site of an existing nodule.
Resources
- Rothstein E, Kohl KS, Ball L, Halperin SA, Halsey N, Hammer SJ, et al. Nodule at injection site as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine. 2004;22;575-585. doi: 10.1016/j.vaccine.2003.09.005
- MVEC: Injection site reactions
Authors: Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute) and Georgina Lewis (Clinical Manager, SAEFVIC, Murdoch Children’s Research Institute)
Reviewed by: Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Katie Butler (MVEC Education Nurse Coordinator) and Rachael McGurie (MVEC Education Nurse Coordinator)
Date: January 2024
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Immunosuppression and vaccines
Background
Individuals who are immunocompromised have a weakened immune system, resulting in a decreased ability to fight infections. There are many different causes of immunocompromise, including having certain medical conditions (e.g. autoimmune diseases, cancer, anatomical or functional asplenia, HIV), being a transplant recipient or advancing age. There are also certain medications that can suppress the immune system, sometimes known or immunosuppressive therapies (e.g. corticosteroids, disease-modifying anti-rheumatic drugs [DMARDs] or cancer therapies).
The term immunosuppression is often used interchangeably with the term immunocompromise. People with a fully functioning immune system can be referred to as immunocompetent.
Immunosuppression and vaccines
Vaccination is particularly important for those who are immunocompromised, due to the increased risk of developing severe disease (which can lead to hospitalisation, intensive care admission or death) if exposed to vaccine-preventable diseases. In people who are immunocompromised, protection from vaccines can be suboptimal as the body is not as easily able to respond to the vaccine. Therefore, additional doses of vaccines may be recommended. Conversely, some vaccines (live-attenuated vaccines) may be contraindicated due to the potential risk of vaccine-related disease.
Taking a thorough patient history prior to vaccination is recommended to determine an individual’s degree of immunocompromise/immunosuppression and to formulate an individualised vaccination strategy.
Vaccination timing
Vaccination may need to be planned with the treating specialist. In some instances, the timing of immunosuppressive therapies may be altered to maximise the response to vaccines. In other circumstances, the intervals between vaccine doses may be altered to accommodate treatment regimes.
In some instances, vaccines can be given pre-emptively to people who anticipate immunosuppression in the future (e.g. a patient undergoing a planned splenectomy should be immunised prior to surgery).
Recommendations
Live-attenuated vaccines must not be given to immunocompromised individuals without consultation with a treating specialist. The following information outlines specific vaccine recommendations for people who are immunosuppressed.
Influenza
Every year, different strains of influenza circulate in the community. Annual vaccines are updated to protect against the strains anticipated to be circulating. People with immunocompromise may be more vulnerable to influenza and associated secondary infections. As such, annual influenza immunisation is recommended and funded for all people with immunosuppression aged over 6 months.
There are precautions relating to influenza vaccines and patients who are receiving treatment with checkpoint inhibitors. Specific information can be found in The Australian Immunisation Handbook.
Pneumococcal
People with immunosuppression have the highest risk of experiencing invasive pneumococcal disease. They are recommended and funded to receive extra pneumococcal vaccine doses in addition to the doses recommended for immunocompetent people. The timing of vaccination, number of doses required, and type of vaccine (s) depend on the person’s age, and their medical and immunisation history.
For more information, refer to MVEC: Pneumococcal
Meningococcal
People receiving certain therapies or with specific medical conditions (particularly those with asplenia) are recommended and funded to receive a primary course of meningococcal B and ACWY vaccines. Depending on the age at which the course is commenced, a primary course for immunocompromised individuals may consist of more doses than a primary course recommended for immunocompetent individuals. Following this, booster doses are recommended for some individuals with specified medical conditions or treatment that increase their risk of invasive meningococcal disease (IMD).
For more information, refer to the MVEC: Meningococcal
Herpes zoster (shingles)
Zoster presents more commonly (and is more likely to present on repeated occasions) in people with immunocompromise compared to immunocompetent people.
Vaccination with a 2-dose course of the vaccine Shingrix is required for adequate protection against zoster. Shingrix is funded on the NIP for people aged over 18 years with history of haemopoietic stem cell transplant, solid organ transplant, blood cancer and advanced or untreated HIV (and for immunocompetent First Nations Australians aged 50 years and over, and other immunocompetent people aged 65 years and over).
Other individuals who are immunocompromised or will soon become immunocompromised can privately purchase a course of Shingrix from 18 years of age. Duration of protection may be limited, so consideration should be given to timing administration to mitigate the greatest risk of disease.
For more information, refer to the MVEC: Zoster
COVID-19
COVID-19 vaccination is strongly recommended for all immunosuppressed individuals aged 6 months and older due to an increased risk of developing severe disease. A 3-dose primary course is recommended for optimal protection (compared with a 2-dose course for those who are immune competent). Following a primary course, booster doses are also recommended for some individuals.
For more information, refer to MVEC: COVID-19
Human papillomavirus (HPV)
People with immunocompromise (with the exception of those with asplenia and hyposplenia) are recommended to receive a 3-dose course of HPV vaccination to ensure adequate protection. This is in contrast to the recommended single dose for immunocompetent individuals aged 9 to 25 years (funded for all adolescents in year 7 of high school).
For more information, refer to MVEC: Human papillomavirus (HPV)
Contraindicated vaccines
Live-attenuated vaccines are contraindicated for most immunocompromised individuals due to the risk of adverse events or vaccine-related disease. In some instances, an alternate inactivated vaccine may be available for use (see table 1).
Table 1: Contraindicated vaccines in immunosuppressed patients and alternative options to consider
| Disease | Contraindicated vaccine brands (live-attenuated vaccines) | Alternate vaccine brands (inactivated vaccines) |
| Japanese encephalitis¥ | Imojev | JEspect |
| MMR (measles-mumps-rubella)^ | Priorix, M-M-R II | N/A |
| MMRV (measles-mumps-rubella-varicella)^ | Priorix-tetra, ProQuad | N/A |
| Mpox¥ | ACAM2000 | JYNNEOS |
| Rotavirus^ | Rotarix | N/A |
| Tuberculosis#¥ | BCG (varying brands) | N/A |
| Typhoid¥ | Vivotif | Typhim, Vivaxim (in combination with hepatitis A) |
| Varicella¥ | Varivax, Varilrix | N/A |
| Yellow fever¥ | Stamaril | N/A |
| Zoster^ | Zostavax | Shingrix |
^routinely administered on the National Immunisation Program (NIP)
#recommended vaccine for select patient group only
¥available vaccine for those at higher risk of infection (e.g. travel)
N/A no alternate vaccine available
Inadvertent administration of a live-attenuated vaccine
If an immunocompromised individual is inadvertently administered a live-attenuated vaccine, prompt action is required. Medical review by an infectious diseases specialist or immunisation expert must be facilitated and the appropriate management commenced (e.g., anti-viral therapy, monitoring etc.).
The vaccine recipient must be informed of the incident and have a clear understanding of its implications, including any signs and symptoms to monitor for. The error must also be reported to the relevant authority to ensure appropriate follow up and support can be provided. In Victoria, this service is SAEFVIC.
If the error occurs out of hours, seek specialist advice from the individual’s treating specialist or an infectious diseases specialist at your local tertiary hospital.
Precautions
Mothers who are receiving immunosuppressive therapy and breastfeeding (or those who received immunosuppressive medication during pregnancy) should seek advice from a Specialist Immunisation Clinic around the safety of live-attenuated vaccines for their child (e.g. oral rotavirus vaccine or BCG).
See MVEC: Immunosuppression in pregnancy and infant vaccine recommendations
Household contacts
Household contacts of immunosuppressed individuals should be up to date with all vaccines and are recommended to receive annual influenza vaccination as well as COVID-19 vaccines.
It is safe for household contacts to receive live-attenuated vaccines (including rotavirus and varicella). Thorough hand hygiene should always be performed when handling soiled nappies of rotavirus vaccine recipients to minimise the risk of vaccine-virus transmission. Any varicella-like blisters that occur on the vaccinee following varicella vaccination should be covered until they crust over.
Other precautions
For further information related to specific conditions and vaccination, refer to the Australian Immunisation Handbook.
Resources
- MVEC: Asplenia
- MVEC: Zoster
- MVEC: Solid organ transplant recipient: pre-transplant immunisation recommendations
- MVEC Cancer immunisation guideline: vaccine recommendations following chemotherapy and haematopoietic stem cell transplant
- MVEC: Immunisation recommendations for the older population
- Australian Immunisation Handbook: Vaccination for people who are immunocompromised
- MVEC eLearning course: Vaccine errors: Prevention, management and open disclosure [for providers]
Authors: Georgina Lewis (Clinical Manager, SAEFVIC, Murdoch Children’s Research Institute), Francesca Machingaifa (MVEC Education Nurse Coordinator) and Rachael McGuire (MVEC Education Nurse Coordinator)
Reviewed: Sally Gordon (MVEC Senior Research Fellow) and Rachael McGuire (MVEC Education Nurse Coordinator)
Date: December 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
Injection site reactions
Background
Injection site reactions (ISRs) are a common localised side effect that can occur following the administration of any injected vaccine. They are an inflammatory response to the injected vaccine. Symptoms of ISRs include swelling, redness (erythema), induration (hardness), pain or itch at or near the injection site.
Less commonly, significant or severe ISRs can present after an injection, extending over a greater area from ‘joint-to-joint’ (e.g. from the shoulder joint to the elbow joint) or ‘crossing joints’ (e.g. swelling that passes over one joint, such as the shoulder or the knee).
High-grade fevers are not typically associated with ISRs.
Diagnosis
A GP, immunisation specialist or immunisation provider can diagnose an ISR based on the clinical symptoms and timeline of presentation.
ISRs typically occur within the first 48 hours following vaccination. Symptoms usually last 1 to 2 days and completely resolve within a week. In some rarer instances ISRs can last for a longer period of time (e.g. 5 to 7 days) or have a delayed onset (3 or more days after vaccination).
Photographic evidence and monitoring the circumference of the affected area over time (e.g. draw around the ISR with a pen to see changes in circumference) can assist in documenting the progression of symptoms and support a diagnosis.
Significant ISRs should not be confused with infections such as cellulitis. Symptoms consistent with cellulitis, such as decreased range of movement, lymphangitis (tracking of erythema), lymphadenopathy (swollen lymph nodes) and high-grade fevers, are not consistent with ISRs.
ISRs are not an allergic response.
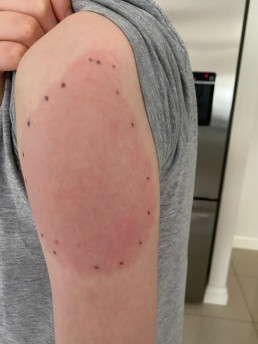
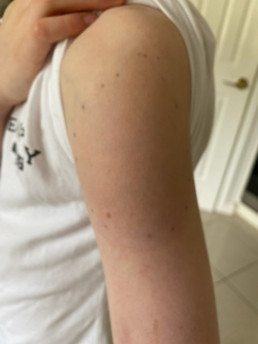
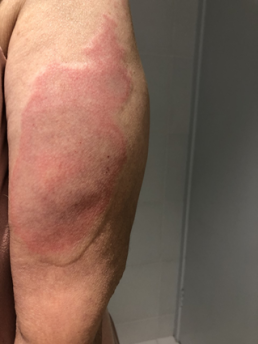
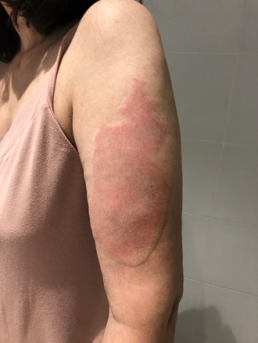
Association and incidence
The true incidence of ISRs is difficult to ascertain as they are often underreported. All injected vaccines have the potential to cause ISRs. However, factors such as the inclusion of adjuvants in the vaccine ingredients, previous exposure to an antigen (through vaccination) and the injection technique used can influence the likelihood of an ISR developing.
Vaccines that contain adjuvants (specific ingredients that are included to evoke stronger immune responses) are often associated with a higher incidence of ISRs. Examples of these vaccines include Shingrix (vaccination against the development of herpes zoster), tetanus-containing vaccines (such as Infanrix hexa and Boostrix), meningococcal vaccines (such as Bexsero), pneumococcal vaccines (Pneumovax 23 and Prevenar 13) and adjuvanted influenza vaccines given to those aged 65 years and over (Fluad Quad and Fluzone High-Dose Quadrivalent).
COVID-19 vaccines and Prevenar 13 are associated with delayed-onset ISRs (occurring more than 3 days after vaccination) when administered to adults 70 years and older.
There is an increased likelihood of ISRs occurring following subsequent doses (primary or boosters) of a particular antigen (e.g. in children aged 18 months and 4 years following diphtheria-tetanus-pertussis boosters, and those receiving subsequent doses of pneumococcal vaccines).
The inadvertent administration of a vaccine into the subcutaneous (SC) tissue, where intramuscular (IM) administration is recommended, has also been associated with an increased incidence of ISR development. Correct injection technique (including route, and appropriate needle size and length) plays an important in mitigating the development of ISRs.
Treatment
ISRs will resolve on their own without intervention. They can generally be managed at home with symptomatic relief such as oral analgesia and applying a cold compress to the affected area. Immobilising the affected limb should be avoided; movement will enhance and assist with lymphatic drainage to improve symptoms.
ISRs are not a sign of allergy or local infection. Therefore, antihistamines, steroids or antibiotics are not required.
Implications for future doses
While ISRs are a common and expected side effect following most injected vaccines, significant or severe ISRs should be reported to SAEFVIC in Victoria.
A previous experience of an ISR is not a contraindication to future doses of the same or any other vaccine. Individuals who have experienced ISRs in the past may experience them again, but they are unlikely to be worse. The benefit of being protected against vaccine-preventable diseases far outweighs the possible risk of developing an ISR. Vaccine recipients who have previously experienced an ISR are encouraged to complete the recommended vaccine schedules.
Resources
- MVEC: Adverse Event Reporting Australia
- MVEC: SAEFVIC
- MVEC: Injection site nodules
- Australian Immunisation Handbook: Common AEFIs and how to manage them
- Australian Immunisation Handbook: Table. Comparison of the effects of diseases and the side effects of vaccines on the NIP
- Australian Immunisation Handbook: Vaccination procedures. After vaccination
- NCIRS: Injection site reactions information sheet
Author: Rachael McGuire (SAEFVIC Research Nurse, Murdoch Children’s Research Institute)
Reviewed by: Melissa Humann (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Rachael McGuire (MVEC Education Nurse Coordinator) and Katie Butler (MVEC Education Nurse Coordinator)
Date: December 2023
Materials in this section are updated as new information and vaccines become available. The Melbourne Vaccine Education Centre (MVEC) staff regularly reviews materials for accuracy.
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.