Immunosuppression in pregnancy and infant vaccine recommendations
背景
Immunosuppressive therapies play an important role in the treatment of many medical conditions. Biologic disease-modifying anti-rheumatic drugs (bDMARDs) are a specific subset of immunosuppressive medications primarily used for the treatment of inflammatory rheumatic diseases. However, they are increasingly being used to treat many other conditions. Examples of bDMARDs include adalimumab, tocilizumab, etanercept, infliximab and rituximab.
If exposed to vaccine-preventable diseases, individuals with suppressed immune systems may have an increased risk of developing severe disease (and of hospitalisation, intensive care admission and death). However, some vaccines (live-attenuated vaccines) may be contraindicated in individuals with immunosuppression due to the potential risk of vaccine-related disease. Additionally, immune response秒 to vaccines may be suboptimal.
Immunosuppression use in pregnancy 和 infant vaccines
Due to the broadened scope of use 的 bDMARDs, including during pregnancy, there is an increasing number of infants exposed to bDMARDs in utero. The effect that this has on an infant’s immune system is not well understood, but there is 这 potential 为了 significant implications regarding the safe use and effectiveness of routine and 额外的 vaccines in infants.
建议
The following guidance outlines recommendations for specific investigations 和 vaccines for infants exposed to maternal immunosuppression (bDMARDs) in utero. This guidance has been developed as a collaboration between MVEC, Queensland Children’s Hospital 和 Royal Brisbane and Women’s Hospital.
Vaccination recommendations for infants exposed to maternal immunosuppression (PDF)
NB: This document provides guidance 在 这 maternal use of bDMARDs only (不是 其他 conventional DMARDs). It outlines the implications for infants 和 does 不是 replace investigations or vaccine recommendations for pregnant women who are receiving/have received bDMARDs.
资源
- Guidance: Vaccination recommendations for infants exposed to maternal immunosuppression (PDF)
- MVEC:免疫抑制和疫苗
- MVEC: Rituximab and immunisation recommendations
- The Society of Hospital Pharmacists in Australia: bDAMRDS quick reference guide
作者: Angela Berkhout (Paediatric Infectious Diseases Physician & General Paediatrician, Children’s Health Queensland), Sophie Wen (Paediatric Infection Specialist, Children’s Health Queensland) , Michael Nissen (Infectious Diseases, Microbiology and Paediatric Consultant, Royal Brisbane and Women’s Hospital), Rachael McGuire (MVEC Education Nurse Coordinator) and Nigel Crawford (Director, SAEFVIC and MVEC, Murdoch Children’s Research Institute)
日期: December 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
特殊免疫服务国际网络
背景
这 国际特殊免疫服务网络 (INSIS) 是疫苗安全监测系统的协调国际合作。通过全球范围内的合作,免疫接种后罕见的严重不良事件(发生在每 10,000 名疫苗接种者中不到 1 人)更有可能被识别、彻底调查和更好地理解。国际领导者是 Karina Top(加拿大)和 Bob Chen(美国)。
目标
INSIS 旨在提高对疫苗安全性的信心。实施标准化的病例定义和方案可以帮助识别与免疫接种后罕见不良事件 (AEFI) 相关的独特分子特征和生物标志物,并提高我们对不良事件遗传基础的理解(例如 血栓形成伴血小板减少综合征 和 心肌炎/心包炎 继 COVID-19 疫苗之后)。通过确定 AEFI 的原因并确定经历 AEFI 的个体风险因素,它可以为最安全的方法提出建议,以对有 AEFI 病史或被确定为经历 AEFI 的高风险人群进行免疫接种。这种更好的理解也将有助于通过 MVEC 等领先网站进行疫苗安全交流和资源开发。
赛维克, 与合作 AEFI-CAN, 领导澳大利亚参与 INSIS。
资金
INSIS 由 流行病防范创新联盟 (CEPI), 这 加拿大卫生研究院 (CIHR) 和 IWK健康中心.
资源
作者: Rachael McGuire(MVEC 教育护士协调员)和 Nigel Crawford(默多克儿童研究所 SAEFVIC 主任)
日期: 3 月 9, 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
免疫性血小板减少症 (ITP)
背景
免疫性血小板减少症,以前也称为特发性血小板减少性紫癜 (ITP),是一种罕见的自身免疫性疾病,患者自身的免疫系统会攻击血小板(血液中发现的细胞,通常有助于血液凝结)。在 ITP 中,身体会产生攻击并破坏血小板的抗体,从而减少血小板数量,并可能导致皮肤瘀伤、针尖红点(瘀点)或出血(例如鼻血或牙龈出血)等症状。有些人根本没有任何症状。
ITP 最常由病毒性疾病引发,发生在症状出现前几周。 ITP 可以是急性(持续少于 6 个月)或慢性(持续超过 6 个月),其中急性 ITP 在儿童中更为常见,慢性 ITP 在成人中更为常见。急性和慢性ITP的症状是相同的。大约每 10,000 名儿童中就有 1 名受到影响 ITP.
有些 ITP 病例是偶然发现的。在某些情况下,ITP 无需任何治疗即可自行消失。然而,当血小板非常低或有出血症状时,可能需要治疗。皮质类固醇和静脉注射免疫球蛋白是最常见的初始治疗形式。
ITP 和麻疹、腮腺炎和风疹疫苗 (MMR)
MMR 疫苗与 ITP 相关,估计每 25,000 次疫苗接种中就有 1 次发生该风险。然而,接种 MMR 疫苗后发生 ITP 的风险远低于自然感染麻疹或风疹的风险。仍然建议有 ITP 病史的患者按照国家免疫计划 (NIP) 接种 MMR 疫苗,因为虽然复发风险很小,但这种风险仍然存在于病毒本身中,重要的是人们受到保护,免受这些病毒的侵害,这些病毒可能导致严重的发病率和死亡率。
ITP 和 COVID-19 疫苗
目前正在调查 COVID-19 疫苗与 ITP 之间的联系。这是因为 MMR 疫苗和 ITP 之间已知的联系,也因为 ITP 可以通过 由 COVID-19 感染引发。苏格兰的一项研究报告了两者之间的联系 Vaxzevria(阿斯利康) 和 ITP,接种疫苗后的发病率高于社区中 ITP 的背景发病率。迄今为止,尚未发现 mRNA COVID-19 疫苗之间存在关联(社区 或者 秒杀)和ITP。澳大利亚正在进行监测和调查。
有 ITP 病史的患者是否应该接种 COVID-19 疫苗?
是的。 COVID-19 疫苗接种对已有 ITP(急性和慢性)的影响尚未得到很好的表征。有限的早期数据表明,疫苗接种可能会使约 10% 接种疫苗后患有慢性 ITP 的患者的血小板减少症恶化。然而,值得注意的是,ITP 最常由病毒引发,如果这些患者感染 COVID-19,ITP 复发或恶化的风险可能比接种疫苗本身的风险更高。因此,建议有 ITP 病史的患者继续接种疫苗,但是,如果疫苗接种后(数天至数周)临床症状恶化,则可能需要监测血小板并升级治疗。
接种一剂 COVID-19 疫苗后出现 ITP 的患者可以接受未来的疫苗吗?
是的。接受 COVID-19 疫苗后出现 ITP 的患者,一旦被告知安全的话,可以接受未来的剂量(包括加强剂量)。应推迟疫苗接种,直至血小板稳定(>50 x 109/L 并停止任何 ITP 治疗 >3 个月)。如果患者继续接受免疫抑制(例如皮质类固醇),他们应该与血液科医生讨论是否继续治疗。
如果患者(> 18 岁)在服用 Vaxzevria(阿斯利康)后患有 ITP,他们应该接受 mRNA 疫苗(例如 Comirnaty 或 Spikevax)或 Nuvaxovid (Novavax) 用于后续剂量。如果患者在注射第一剂 mRNA 疫苗或 Nuvaxovid 后患有 ITP,则应继续注射第二剂同一疫苗,因为迄今为止尚未发现这些疫苗与 ITP 之间存在关联。无论疫苗品牌如何,都存在复发风险,但 COVID-19 疾病本身的复发风险更高。接种第 2 剂疫苗后应进行密切监测,以确保血小板不再下降。
ITP 和其他疫苗
有一些小型研究或病例报告表明,ITP 和其他疫苗(例如流感、HPV、脊髓灰质炎和肺炎球菌疫苗)的风险可能会增加。然而,迄今为止,除了上述 MMR 和 Vaxzevria(阿斯利康)疫苗外,还没有其他疫苗被证明与 ITP 相关。有 ITP 病史的人可以安全地根据需要接种所有常规 NIP 和旅行疫苗。
资源
- Miller E、Waight P、Farrington CP、Andrews N、Stowe J、Taylor B。特发性血小板减少性紫癜和 MMR 疫苗。阿奇·迪斯·孩子。 2001;84(3):227-9
- Black C,Kaye JA,Jick H。 MMR 疫苗和特发性血小板减少性紫癜。 Br J 临床药理学。 2003;55(1):107-11
- Bhattacharjee S、Banerjee M。继发于 COVID-19 的免疫性血小板减少症:系统评价。 SN Compr 临床医学。 2020年:1-11
- Simpson CR、Shi T、Vasileiou E、Katikireddi SV、Kerr S、Moore E 等。苏格兰首剂 ChAdOx1 和 BNT162b2 COVID-19 疫苗以及血小板减少、血栓栓塞和出血事件。纳特医学。 2021;27(7):1290-7
- Rinaldi M、Perricone C、Ortega-Hernandez OD、Perricone R、Shoenfeld Y。免疫性血小板减少性紫癜:感染与疫苗之间的自身免疫交叉联系。狼疮。 2014;23(6):554-67
- Garbe E、Andersohn F、Bronder E、Salama A、Klimpel A、Thomae M 等。药物引起的免疫性血小板减少症:柏林病例对照监测研究的结果。欧洲临床药理学杂志。 2012;68(5):821-32
作者: Sally Gordon(卫生部 VicSIS 经理)、Paul Monagle(皇家儿童医院临床血液学家)、Francesca Machingaifa(MVEC 教育护士协调员)、Rachael McGuire(MVEC 教育护士协调员)和 Nigel Crawford(默多克儿童研究所 SAEFVIC 主任) )
审核人: Paul Monagle(皇家儿童医院临床血液学家)、Nigel Crawford(默多克儿童研究所 SAEFVIC 主任)和 Rachael McGuire(MVEC 教育护士协调员)
日期: 7 月 21, 2022
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
流行性感冒
什么是流行性感冒?
流行性感冒(流感)病毒是单链RNA正粘病毒,可导致呼吸道急性病毒感染。通常按照引发感染的流感病毒类型划分感染类型,一般为A型、B型或C型。A型和B型流感更常引发严重疾病。根据表面抗原的不同,A型流感还可以进一步划分为多个亚型。
流感症状
流感疾病的潜伏期为1至4天,典型症状包括发烧、头痛、肌痛(肌肉酸痛)、嗜睡(疲倦)、卡他性鼻炎(流鼻涕)、喉咙痛和咳嗽。 还可能出现恶心、呕吐、腹泻等胃肠道症状。 感染流感的儿童通常也会出现以下症状哮吼症症状。
大部分流感感染会在2至7天内缓解。不过,中耳炎(耳部感染)、继发性细菌性肺炎(肺部感染)和脑炎(脑部炎症)等并发症会延长病情和疾病结局。
传播途径
流感的传染性极高。这种疾病是通过呼吸道飞沫、气溶胶或通过直接接触受感染患者的呼吸道分泌物而传播。
流行病学
流感疾病的发生形式可以是散发病例、流行病或者大流行。在温带地区,流感通常在冬季爆发;而在热带地区,流感则在不同时间爆发。
老年人护理机构、医疗护理机构以及儿童护理中心通常被认为是爆发流感的高风险区域。
在澳大利亚,流感在孕妇、5岁以下儿童、65岁以上老年人、有基础疾病的人以及原住民和托雷斯海峡岛民等人群中有较高的发病率和死亡率。
预防措施
流感疫苗可供6个月以上希望预防流感及其并发症的人接种。由于流感病毒的传播毒株每年都在变化,建议每年接种疫苗,以提供最有效的疾病防护。
2024年,国家免疫接种项目(NIP)为高危人群免费提供流感疫苗,该人群包括:
5至64岁没有资格获得免费疫苗的人士可以通过市政府、全科医生和药店自行购买疫苗。 理事会, 全科医生和 药房.
疫苗平台
在澳大利亚提供的是灭活流感疫苗,它们不会复制并引发流感疾病。按照生产工艺的不同,这些疫苗可以是细胞培养或鸡胚培养疫苗.
传统的流感疫苗是通过在鸡蛋中培养流感病毒制成的。细胞培养的流感疫苗是通过在动物细胞系(犬肾)中培养流感病毒制成的。澳大利亚免疫技术咨询小组(ATAGI)对细胞培养流感疫苗和传统鸡胚培养疫苗没有特别偏好(对鸡蛋过敏/有鸡蛋过敏性反应的个人可以安全地接种鸡胚培养流感疫苗——见下方的常见问题)。
Table 1: T表1:2024年季节性流感疫苗中的流感病毒株
虽然世界卫生组织(WHO)和澳大利亚流感疫苗委员会(AIVC)认识到B Yamagata毒株已经好几年没有传播了,没有必要将其纳入年度疫苗,但它仍被纳入了2024年流感疫苗。不必担心这样做是否安全 WHO和AIVC支持将其纳入 (参考下文资源)
佐剂和高剂量疫苗
由于效果逐渐下降 老年人的免疫系统 这一过程被称为免疫衰老),接种标准流感疫苗后的免疫力可能无法达到最佳水平。此外,65岁以上人群流感疾病负担和相关并发症(包括肺炎和死亡)的发生率最高。因此,为了增加免疫反应,佐剂(Fluad Quad)或高剂量(Fluzone High-dose Quad)流感疫苗是老年人群的首选疫苗类型。
表2:根据年龄推荐的2024年流感疫苗品牌
| 年龄组别 | 疫苗品牌和剂量 | |||||||
| Vaxigrip Tetra® (0.5 毫升) | Fluarix® Tetra (0.5 毫升) | Afluria® Quad (0.5 毫升) | FluQuadri® (0.5 毫升) | Influvac® Tetra (0.5 毫升) | Flucelvax® Quad (0.5 毫升) | Fluad® Quad (0.5 毫升) | Fluzone High-Dose Quad (0.7 毫升) | |
| < 6 个月 | ||||||||
| 6 个月 - < 2 岁 | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ||||
| ≥2- < 5 岁 | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ✓*^ | |||
| ≥5- < 60 岁 | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ✓*^ | ||
| ≥60- < 65 岁 | ✓^ | ✓^ | ✓^ | ✓^ | ✓^ | ✓^ | ✓# | |
| ≥ 65 岁 | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓β^# | ✓# | ✓# |
* 2剂,年龄在9岁以下儿童,第一年接种了流感疫苗后,应间隔至少4周后再接种,之后的每年建议接种一剂。
ΩΩ NIP资金仅用于原住民、孕妇和有医疗风险因素的人
#建议65岁以上的成年人以佐剂或高剂量四价流感疫苗为首选疫苗。
ββFluarix tetra/FluQuadri/Afluria Quad/Vaxigrip tetra/Influvac tetra/Fflucelvax Quad已注册用于65岁以上的人群,但佐剂或高剂量疫苗是该年龄组的首选疫苗。
^无论是否因免疫抑制而接种过流感疫苗,建议在实体器官移植(SOT) 或造血 血干细胞移植(HSCT) 免疫抑制后的第一年接种2剂。而接受佐剂或高剂量流感疫苗的个人,建议只接种1剂。
阴影方框表示给有资格获得NIP资助的人士提供的疫苗
阴影方框表示未注册用于该年龄组别
阴影方框表示佐剂或高剂量疫苗。
预期的副作用
接种疫苗后常见副作用包括注射部位出现疼痛、红斑和浮肿,以及发烧、疲倦乏力和肌肉酸痛。相关症状通常在接种疫苗后24至48小时内出现。
细胞培养流感疫苗和传统的鸡胚培养流感疫苗具有相似的副作用特征。与标准的流感疫苗制剂相比,接种佐剂四价疫苗制剂后出现副作用的几率略高。
常见问题
什么时候接种流感疫苗最好?
对于所有年龄在6个月及以上的人士,建议每年在流感季节开始前接种疫苗。在澳大利亚,流行性感冒疾病的高峰期通常是6月到9月。不过,流感季节外也有可能会出现病例,也确实有病例发生。接种疫苗后的头3到4个月,疫苗对流感的预防保护作用最佳。在流感季节,任何时候接种疫苗都不晚。
孕妇在怀孕的任何阶段接种流感疫苗都是安全的。如果孕期跨越两个流感季节,建议某些孕妇接种2次流感疫苗,每年一次。
健康人士是否需要接种流感疫苗?
流感有可能发展成为非常严重的疾病,并导致住院和死亡。即使在病情及其并发症都不是很严重的情况下,也会给患者带来极大的不便,不仅需要与全科医生面诊并且支付药物治疗,还需要请病假休息或照顾生病的孩子。
在某些情况下,受感染的人也许病情不严重,但会将疾病传染给其他人。如果传给那些年龄太小而无法接种疫苗,或者具有较高并发症风险的人,情况会变得很严重。
如果某个人今年确诊流感,那么是否仍然建议他们接种流感疫苗?他们又应当在什么时候接种?
仍建议有确诊流感感染史的人接种疫苗,因为疫苗可以预防多种流感疾病。患者一旦康复就可以接种流感疫苗。
流感疫苗能和其他疫苗同时接种吗?
是的,流感疫苗可以与任何其他疫苗在同一天同时接种。这包括减毒活疫苗(如麻疹和水痘)和孕期百日咳疫苗。 怀孕.
如果患者在2024年初流感季节结束时接种了2023年流感疫苗,他们还需要接种2024年流感疫苗吗?
是的,仍建议接种2024年流感疫苗,以预防被今年流行毒株感染。建议至少间隔4周。
对于9岁以下、去年首次接种流感疫苗但只接种了1剂的儿童,今年他们需要接种多少剂?
在这种情况下,只需要接种1剂。对于9岁以下、处于接种疫苗第一年的儿童,建议接种2剂流感疫苗。不过,如果无意中错过第二剂,也无需补种,在未来的年份里只需接种1剂即可。
流感疫苗对于过敏人群而言是安全的吗?
根据对鸡蛋过敏(包括鸡蛋过敏性反应)人士和无鸡蛋过敏人士的前瞻性和回顾性研究,鸡蛋过敏并不增加对流感疫苗过敏性反应的风险。鸡胚培养的流感疫苗可以在社区免疫接种诊所(有或没有医疗从业人员的直接监督均可)、全科医生诊室或免疫接种诊所进行单剂接种,然后按建议观察15分钟。这个患者群体,不需要以细胞培养的流感疫苗为首选。
2024年NIP提供的所有流感疫苗都不含乳胶,这意味着乳胶过敏的人可以安全地接种疫苗。
如需进一步查询流感疫苗接种的问题,请通过我们的免疫接种支持服务联系我们。
资源
- ATAGI关于2024年季节性流感疫苗管理的声明
- 澳大利亚药品管理局(TGA):AIVC对2024年澳大利亚流感疫苗成分的建议
- 卫生部(Department of Health):季节性流感疫苗
- Department of Health:2024年流感疫苗接种——对医务人员的项目建议
- 澳大利亚免疫手册(Australian Immunisation Handbook):流感
- 澳亚临床免疫与过敏协会(Australasian Society of Clinical Immunology and Allergy):鸡蛋过敏人士的疫苗接种
- 墨尔本疫苗教育中心(MVEC):孕期孕妇疫苗接种
作者::Nigel Crawford副教授(默多克儿童研究所(Murdoch Children’s Research Institute)SAEFVIC主任)、Rachael McGuire(Murdoch Children’s Research Institute SAEFVIC研究护士)、Georgina Lewis(Murdoch Children’s Research Institute临床经理)和Mel Addison(Murdoch Children’s Research Institute SAEFVIC研究护士)。
审阅者:Rachael McGuire(MVEC教育护士协调员).
日期: 2024年3月
本节内的材料将随着新信息的出现而进行更新。MVEC职员定期审阅材料的准确性.
本站点的信息并非是针对您或您家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,请务必咨询医疗保健专业人士。
针对老年人口的免疫建议
老年人口的疫苗接种需要考虑多种因素。随着人们年龄的增长,免疫系统会逐渐衰退(称为免疫衰老),影响免疫系统对新感染的反应以及长期免疫记忆的有效性。正是由于这个原因,一些疫苗是专门为老年人群设计的,旨在通过使用更高免疫原性的制剂或含有佐剂来增强免疫反应。由于老龄化人口中多种合并症的患病率不断增加,提供最佳保护也可能变得复杂。特定的医疗状况或靶向治疗(例如慢性肾病、癌症化疗等)也可能导致老年人更容易受到感染及其并发症。此外,依靠患者回忆以及对老年人群推荐疫苗缺乏认识,可能会导致错过疫苗或注射额外不必要的剂量。
建议老年人接种多种疫苗,如下所述。
带状疱疹疫苗
带状疱疹 是由水痘病毒重新激活引起的,大约 20-30% 的人一生中会发生这种情况。老年人(> 70 岁)比年轻人更容易在带状疱疹感染后出现带状疱疹后神经痛 (PHN)。 PHN 是一种慢性神经性疼痛,在 80 岁以上诊断出的带状疱疹病例中,有四分之一会受到影响。它可能持续数月至数年,难以控制疼痛,影响生活质量。
澳大利亚目前有 2 种疫苗可用于预防带状疱疹:
- Zostavax®- 减毒活疫苗
- Shingrix® - 一种带佐剂的重组水痘带状疱疹病毒糖蛋白 E (gE) 亚基(非活)疫苗
Zostavax®
Zostavax® 已被证明可将带状疱疹的发生率降低高达 50%,并且 66% ≥ 60 岁人群中 PHN 的发生率。目前,该计划由针对 70 岁人群的国家免疫计划 (NIP) 资助,还为 71-79 岁人群的补种计划提供资助(直至 2023 年 10 月)。由于它是减毒活疫苗,因此 禁忌 用于免疫抑制或正在服用免疫抑制药物(例如利妥昔单抗、硫唑嘌呤、泼尼松龙、化疗等)的患者。在施用 Zostavax® 之前,重要的是要详细了解患者病史以确定是否适合免疫接种。
新格里克斯®
在预防带状疱疹方面,Shingrix® 比 Zostavax® 更受青睐,因为其功效更高,尤其是在老年人群中。对于年龄 ≥ 50 岁的人群,Shingrix® 为免疫功能正常的个体提供 97% 保护,防止带状疱疹,并为年龄 > 70 岁的人群提供 91% 保护。临床试验证明了疫苗接种后长达 4 年的高效效果,免疫原性数据表明这种效果可能会持续超过 10 年。
Shingrix® 已注册用于 50 岁以上的成年人。它只能通过私人处方获得,目前供应有限。它是一种非活疫苗,因此 能 安全地给予免疫功能低下的个体。 ATAGI 建议在接种 COVID-19 疫苗和 Shingrix® 之间间隔 7 天,并建议不要在同一天同时接种 FluadQuad 和 Shingrix®。
可以通过审查提供进一步的指导 澳大利亚免疫手册:表格。活带状疱疹疫苗 (Zostavax) 筛查禁忌症 或在免疫接种前联系 SAEFVIC。
肺炎球菌疫苗
侵袭性肺炎球菌疾病 (IPD) 可表现为脑膜炎、肺炎和菌血症,病情严重需要住院治疗,导致严重发病甚至死亡。老年人(以及婴儿)患 IPD 的风险最高。成人肺炎球菌疫苗的建议根据年龄和健康状况而有所不同[请参阅 ATAGI 针对 2020 年 7 月 1 日起针对高危人群的疫苗接种建议的临床建议]。目前 NIP 为以下人群免费提供肺炎球菌疫苗:
- 无风险状况的原住民和托雷斯海峡岛民成年人 – 50 岁时服用 1 剂 Prevenar 13®,8 周后服用 2 剂 Pneumovax® 23,间隔 5 年服用
- 无风险状况的非土著成年人 – 70 岁以上时服用 1 剂 Prevenar 13®
- 被诊断患有危险状况的非土著青少年/成人 – 诊断时服用 1 剂 Prevenar 13®,随后服用 2 剂 Pneumovax® 23,间隔 5 年服用
对于成人,70 岁以上注射 Prevenar 13® 剂量后 3 天以上可能会出现注射部位反应,特别是对于那些之前接受过注射的患者 肺炎疫苗®23。先前接种肺炎球菌疫苗后出现大面积局部注射部位反应的病史并不构成进一步接种疫苗的禁忌症。
参考 MVEC:肺炎球菌 了解更多信息。
流感疫苗
对于老年人和患有某些疾病的人(例如慢性肺病、心脏病、免疫抑制), 流感疾病 可导致严重的发病率和死亡率。强烈鼓励每年接种流感疫苗,并且 NIP 为 65 岁以上和/或患有某些疾病的成年人免费提供疫苗。由于常规流感疫苗的免疫反应减弱,年龄≥65岁的人应接种免疫原性较高的流感疫苗。
参考 MVEC:流感 有关品牌和剂量的具体信息。
2019冠状病毒病疫苗
老年人和患有合并症(例如高血压、糖尿病、慢性肺病等)的人如果感染,更有可能患上严重的 COVID-19 疾病。在 80 岁以上患有 COVID-19 疾病的人中,大约三分之一的人会死于该病。
COVID-19 疫苗接种要求免疫功能正常的个体接种 2 剂初级疗程,或患有免疫缺陷的个体接种 3 剂初级疗程 免疫妥协。初级疗程后应在 ≥ 3 个月后进行加强剂量,并 进一步的“冬季加强剂”剂量 对于选定的个人,≥ 3 个月后。
有关老年人 COVID-19 疫苗接种的更多信息,请参阅 COVID-19 疫苗接种 – 针对体弱老年人(包括居住老年护理机构的老年人)的 COVID-19 疫苗接种决策指南。
居住老年护理机构 (RACF) 居民的注意事项
虽然应尽一切努力为 RACF 居民提供疫苗接种,使其面临 COVID-19 和流感等疫苗可预防疾病的风险,但监测免疫接种后的不良事件 (AEFI) 也很重要。由于认知障碍的发生率很高,老年居民可能没有能力自我报告任何副作用。接种疫苗后 5 天内出现的任何 AEFI 应报告给 赛维克。监测老年人在身体不适时出现的非特异性症状非常重要,例如跌倒、谵妄、功能下降、食欲下降/丧失或情绪/行为变化。
有关 RACF 居民接种疫苗后可能需要的额外护理和症状管理的更多信息,请参阅 维多利亚州住宅老年护理机构居民的疫苗接种护理指南.
向澳大利亚免疫登记处 (AIR) 报告
这 空气 提供所有疫苗剂量、接种日期以及使用的具体品牌的记录。自 2016 年以来,任何年龄段的澳大利亚人接种的疫苗均已记录在 AIR 上。患者回忆(尤其是老年人群)并不可靠,因此准确维护和定期审查免疫记录非常重要。
从 2021 年 3 月起,新立法生效,强制要求向 AIR 报告疫苗。这包括所有 COVID-19 疫苗、流感疫苗和所有国家免疫计划疫苗。
资源
- 澳大利亚政府卫生部:老年人免疫接种
- MVEC:流感
- MVEC:带状疱疹
- MVEC:肺炎球菌疾病和疫苗
- MVEC:COVID-19 疫苗:常见问题解答
- MVEC:澳大利亚免疫登记
- COVID-19 疫苗接种 – 针对体弱老年人(包括居住老年护理机构的老年人)的 COVID-19 疫苗接种决策指南
- 维多利亚州住宅老年护理机构居民的疫苗接种护理指南
- Aw D. Silva B. 和 Palmer D. 免疫衰老:人口老龄化面临的新挑战 免疫学 2007 年 4 月; 120(4):435–446
- Gustafson C. Kim C. Weyand C. Goronzy J 免疫老化对疫苗反应的影响 过敏与临床免疫学杂志 2020 年 5 月; 145(5):1309–1321
作者: Daryl Cheng(皇家儿童医院儿科医生)、Francesca Machingaifa(MVEC 教育护士协调员)和 Rachael McGuire(MVEC 教育护士协调员)
审核人: Francesca Machingaifa(MVEC 教育护士协调员)
日期: 8 月 5, 2022
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
识别不同肤色的 AEFI
背景
在识别免疫接种后的不良事件 (AEFI) 时,评估皮肤的临床体征和症状非常重要。大多数皮肤病学评估指南通常提到浅肤色患者的症状表现。
皮肤是体内最复杂的器官之一。它由 3 层组成,每层执行不同的角色。人的皮肤颜色受到位于表皮(皮肤外层)的黑素细胞产生的黑色素(天然色素)量的影响,深色皮肤比浅色皮肤含有更多黑色素。黑色素的量会影响患者皮肤上 AEFI 的外观,因此 AEFI,例如苍白、发绀、红斑和荨麻疹,在不同肤色下可能会出现不同的情况。这可能给免疫接种提供者及时识别 AEFI 症状带来挑战。
苍白
苍白是指皮肤、甲床和粘膜的苍白外观。苍白并不总是疾病的症状。
深色皮肤的苍白可能很难察觉,可能呈灰白色或灰色。棕色皮肤的皮肤会呈现出更黄的颜色。识别较深肤色苍白的另一种方法是评估可能显得更苍白的手掌表面。
免疫接种后,可在血管迷走性晕厥(昏厥)和 低渗性低反应发作(HHE).
发绀
发绀是血液中氧气减少的症状。紫绀有两种类型:
- 周围发绀,见于四肢,包括手、指尖和脚。
- 中枢性紫绀出现在身体的中央部位,包括头部、躯干和粘膜,通常更为严重。
对于肤色较浅的人,紫绀会呈现蓝/紫色调。对于自然黄皮肤的患者,紫绀可能会导致灰绿色外观。对于肤色较深的人,紫绀可能更难以评估,并且可能表现为灰色或白色。
免疫接种后,在环境中可能会出现紫绀 赫赫、呼吸暂停、屏气发作或高烧。
红斑
红斑是指由浅表血管扩张和血流量增加引起的皮肤红色外观。它常与皮肤外伤、炎症、感染或皮疹一起发生。
浅色皮肤上的红斑清晰可见。在一些深色肤色的皮肤上,它可能会呈现红色或导致紫色变色,但在非常黑的皮肤上很难看到。评估受影响部位或区域的其他体征,包括发热、肿胀或硬结,有助于红斑的临床评估和诊断。
免疫接种后,红斑可能广泛分布或 位于注射部位.
荨麻疹
当皮肤内的肥大细胞释放组胺刺激神经末梢并导致局部血管扩张和渗漏液体时,就会发生荨麻疹或荨麻疹。它们可以出现在身体的任何地方,并且本质上可能是短暂的。原因通常未知,但可能与感染或过敏有关。
在浅色皮肤上,荨麻疹表现为发痒、凸起的红色伤痕。它们通常有一个白色的中心或风团,看起来像蚊子叮咬的地方,周围有红斑环。在深色皮肤中,荨麻疹表现为凸起的肿块,但皮肤颜色的变化可能不那么明显。
免疫接种后,荨麻疹可能发生在身体的任何部位,并且可能发生在有环境的地方。 过敏反应。
资源
图片
其他资源
- DermNetNZ:民族皮肤病学
- 澳大利亚临床免疫学和过敏学会:荨麻疹
- Ortonne, J. 正常和异常肤色,Annales de Dermatologie et de Venereologie 2021 年 12 月 (139):S125-S129
- Sommers, M. 颜色意识:患者评估的必备条件,美国护士,2011 年 1 月
- 皮肤科医生:识别有色皮肤的红斑
作者: Georgina Lewis(默多克儿童研究所 SAEFVIC 临床经理)、Mel Addison(默多克儿童研究所 SAEFVIC 研究护士)、Francesca Machingaifa(默多克儿童研究所 SAEFVIC 研究护士)和 Rachael McGuire(默多克儿童研究所 SAEFVIC 研究护士) )
审阅者:Rachael McGuire(MVEC教育护士协调员)
日期: 5 月 31, 2022
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
本站点的信息并非针对你个人健康或你家人个人健康的特定、专业的医疗建议。对于医疗方面的问题,包括有关免疫接种、药物治疗和其他治疗的决定,你务必咨询医疗保健专业人士。
注射部位结节
背景
The development of an injection site nodule (ISN) is a rare adverse event following immunisation (AEFI). Nodules can occur following administration of any injected vaccine. They usually present in the days or weeks following immunisation. ISNs are most often reported following vaccines given in infancy or childhood. An ISN usually persists for weeks or months. Very rarely, a nodule may persist for years. ISNs are most often asymptomatic, but may be intermittently tender, itchy, or show overlying skin changes (e.g. flaky skin). They generally resolve spontaneously, without treatment or investigation.
诊断
ISNs are defined as a firm, discrete or well-demarcated soft-tissue lump at the site of vaccination, in the absence of heat, erythema (redness) or signs of abscess (e.g. pus, pain). They are often described by parents and caregivers as a pea-size lump under the skin.
Diagnosis is generally based on a healthcare provider’s clinical assessment. Although not routinely recommended, some clinicians may request an ultrasound of the area to confirm or support the diagnosis, and to exclude alternative diagnoses.
ISNs that last for months or years, with symptoms, are referred to as ‘persisting subcutaneous nodules’ (subcutaneous meaning under the skin). Itch is generally the concerning symptom that motivates people to seek medical attention. Ongoing scratching can alter the appearance of the skin leading to excoriation (irritated skin), hair growth and pigmentation changes.
Intensified itching and an increase in size of the nodule can occur during the course of a viral illness, or following subsequent vaccinations administered at a different anatomical site.
Association and incidence
It is unclear what causes an ISN. Factors identified as possibly contributing to ISNs include administration technique, vaccine components (including adjuvants, such as aluminium), patient predisposition and normal immune-mediated responses.
There is limited data on ISN incidence rates. Some estimates are available based on small, local cohorts.
The following references provide additional context on the possible causes and incidence of ISNs:
- Silcock R, Crawford NW, Selvaraj J, McMinn A, Danchin M, Lazzaro T, et al. Subcutaneous nodules following immunization in children; in Victoria, Australia from 2007 to 2016. 疫苗. 2020 Mar 5;38:3169–3177. doi:10.1016/j.vaccine.2019.12.066
- Silcock R, Crawford NW, Perrett KP. Subcutaneous nodules: an important adverse event following immunization. Expert Rev Vaccines. 2019 Mar 11;18(4);405-410. doi:10.1080/14760584.2019.1586540
- Bergfors E, Lundmark K, Nyström Kronander U. A child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines. BMJ Case Rep. 2013;2013:bcr2012007779. Published 2013 Jan 24. doi:10.1136/bcr-2012-007779
治疗
A conservative approach to treatment is recommended; ISNs generally resolve on their own without intervention. On some occasions, health professionals might advise topical corticosteroids to treat the itch or skin changes, and dressings to protect the area from vigorous scratching. Rarely, excision of the nodule may be considered by a specialist, based on nature and severity of symptoms.
Any AEFI should be reported to the vaccine safety service in your state. In Victoria, reports can be made to 赛维克.
对未来剂量的影响
It is recommended that people who experience ISNs continue to receive future vaccines according to the immunisation schedule. The history of, or presence of, a nodule is not a contraindication to future vaccines.
Ensure 正确的疫苗接种 for both intramuscular (IM) and subcutaneous (SC) vaccines. For IM vaccines, consider deep IM injection to minimise the risk of potential recurrence of a nodule. Where possible, avoid vaccination at a site of an existing nodule.
资源
- Rothstein E, Kohl KS, Ball L, Halperin SA, Halsey N, Hammer SJ, et al. Nodule at injection site as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. 疫苗. 2004;22;575-585. doi: 10.1016/j.vaccine.2003.09.005
- MVEC:注射部位反应
作者: Mel Addison(默多克儿童研究所 SAEFVIC 研究护士)、Rachael McGuire(默多克儿童研究所 SAEFVIC 研究护士)和 Georgina Lewis(默多克儿童研究所 SAEFVIC 临床经理)
审核人: Mel Addison (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Katie Butler (MVEC Education Nurse Coordinator) and Rachael McGurie (MVEC Education Nurse Coordinator)
日期: January 2024
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
免疫抑制和疫苗
背景
Individuals who are immunocompromised have a weakened immune system, resulting in a decreased ability to fight infections. There are many different causes of immunocompromise, including having certain medical conditions (e.g. autoimmune diseases, cancer, anatomical or functional asplenia, HIV), being a transplant recipient or advancing age. There are also certain medications that can suppress the immune system, sometimes known or immunosuppressive therapies (e.g. corticosteroids, disease-modifying anti-rheumatic drugs [DMARDs] or cancer therapies).
The term immunosuppression is often used interchangeably with the term immunocompromise. People with a fully functioning immune system can be referred to as immunocompetent.
免疫抑制和疫苗
Vaccination is particularly important for those who are immunocompromised, due to the increased risk of developing severe disease (which can lead to hospitalisation, intensive care admission or death) if exposed to vaccine-preventable diseases. In people who are immunocompromised, protection from vaccines can be suboptimal as the body is not as easily able to respond to the vaccine. Therefore, additional doses of vaccines may be recommended. Conversely, some vaccines (live-attenuated vaccines) may be contraindicated due to the potential risk of vaccine-related disease.
Taking a thorough patient history prior to vaccination is recommended to determine an individual’s degree of immunocompromise/immunosuppression and to formulate an individualised vaccination strategy.
疫苗接种时机
Vaccination may need to be planned with the treating specialist. In some instances, the timing of immunosuppressive therapies may be altered to maximise the response to vaccines. In other circumstances, the intervals between vaccine doses may be altered to accommodate treatment regimes.
In some instances, vaccines can be given pre-emptively to people who anticipate immunosuppression in the future (e.g. a patient undergoing a planned splenectomy should be immunised prior to surgery).
建议
Live-attenuated vaccines must not be given to immunocompromised individuals without consultation with a treating specialist. The following 我nformation outlines 秒pecific 疫苗 recommendations 为了 people who 是 immunosuppress编辑.
流行性感冒
Every year, different strains of 流感 circulate in the community. Annual vaccines are updated to protect against the strains anticipated to be circulating. People with immunocompromise may be more vulnerable to influenza and associated secondary infections. As such, annual influenza immunisation is recommended and funded for all people with immunosuppression aged over 6 months.
There are precautions relating to influenza vaccines and patients who are receiving treatment with checkpoint inhibitors. Specific information can be found in The Australian Immunisation Handbook.
肺炎球菌
People with immunosuppression have the highest risk of experiencing invasive 肺炎球菌 disease. They are recommended and funded to receive extra pneumococcal vaccine doses in addition to the doses recommended for immunocompetent people. The timing of vaccination, number of doses required, and type of vaccine (s) depend on the person’s age, and their medical and immunisation history.
For more information, refer to MVEC:肺炎球菌
脑膜炎球菌
People receiving certain therapies or with specific medical conditions (particularly those with 无脾) are recommended and funded to receive a primary course of meningococcal B and ACWY vaccines. Depending on the age at which the course is commenced, a primary course for immunocompromised individuals may consist of more doses than a primary course recommended for immunocompetent individuals. Following this, booster doses are recommended for some individuals with specified medical conditions or treatment that increase their risk of invasive meningococcal disease (IMD).
Herpes zoster (shingles)
Zoster presents more commonly (and is more likely to present on repeated occasions) in people with immunocompromise compared to immunocompetent people.
Vaccination with a 2-dose course of the vaccine Shingrix is required for adequate protection against zoster. Shingrix is funded on the NIP for people aged over 18 years with history of haemopoietic stem cell transplant, solid organ transplant, blood cancer and advanced or untreated HIV (and for immunocompetent First Nations Australians aged 50 years and over, and other immunocompetent people aged 65 years and over).
Other individuals who are immunocompromised or will soon become immunocompromised can privately purchase a course of Shingrix from 18 years of age. Duration of protection may be limited, so consideration should be given to timing administration to mitigate the greatest risk of disease.
For more information, refer to the MVEC:带状疱疹
新冠肺炎
COVID-19 vaccination is strongly recommended for all immunosuppressed individuals aged 6 months and older due to an increased risk of developing severe disease. A 3剂初级课程 建议使用以获得最佳保护(与免疫能力强的人的 2 剂疗程相比)。完成初级课程后, 加强剂量 are also recommended for some individuals.
For more information, refer to MVEC:COVID-19
人乳头瘤病毒 (HPV)
People with immunocompromise (with the exception of those with asplenia and hyposplenia) are recommended to receive a 3-dose course of HPV vaccination to ensure adequate protection. This is in contrast to the recommended single dose for immunocompetent individuals aged 9 to 25 years (funded for all adolescents in year 7 of high school).
For more information, refer to MVEC: Human papillomavirus (HPV)
禁忌疫苗
减毒活疫苗 are contraindicated for most immunocompromised individuals due to the risk of adverse events or vaccine-related disease. In some instances, an alternate inactivated vaccine may be available for use (see table 1).
表 1:免疫抑制患者的禁忌疫苗和需要考虑的替代方案
| 疾病 | 禁忌疫苗品牌(减毒活疫苗) | 替代疫苗品牌(灭活疫苗) |
| 日本脑炎¥ | 伊莫耶夫 | 杰派克 |
| MMR(麻疹-腮腺炎-风疹)^ | Priorix, M-M-R II | 不适用 |
| MMRV(麻疹-腮腺炎-风疹-水痘)^ | Priorix-tetra, ProQuad | 不适用 |
| 痘痘¥ | ACAM2000 | 吉尼奥斯 |
| 轮状病毒^ | Rotarix | 不适用 |
| 结核病#¥ | BCG(不同品牌) | 不适用 |
| 伤寒¥ | Vivotif | Typhim, Vivaxim (in combination with hepatitis A) |
| 水痘¥ | Varivax, Varilrix | 不适用 |
| 黄热病¥ | Stamaril | 不适用 |
| 带状疱疹^ | Zostavax | Shingrix |
^国家免疫计划 (NIP) 的常规管理
#仅针对特定患者组推荐的疫苗
¥available vaccine for those at higher risk of infection (e.g. travel)
不适用 没有可用的替代疫苗
无意中注射了减毒活疫苗
If an immunocompromised individual is inadvertently administered a live-attenuated vaccine, 迅速行动 is required. Medical review by an infectious diseases specialist or immunisation expert must be facilitated and the appropriate management commenced (e.g., anti-viral therapy, monitoring etc.).
The vaccine recipient must be informed of the incident and have a clear understanding of its implications, including any signs and symptoms to monitor for. The error must also be reported to the relevant authority to ensure appropriate follow up and support can be provided. In Victoria, this service is 赛维克.
If the error occurs out of hours, seek specialist advice from the individual’s treating specialist or an infectious diseases specialist at your local tertiary hospital.
防范措施
Mothers who are receiving immunosuppressive therapy and breastfeeding (or those who received immunosuppressive medication during pregnancy) should seek advice from a Specialist Immunisation Clinic around the safety of live-attenuated vaccines for their child (e.g. oral rotavirus vaccine or BCG).
See 微血管内皮细胞: Immunosuppression in pregnancy and infant vaccine recommendations
家庭联系人
免疫抑制个体的家庭接触者应及时接种所有疫苗,并建议每年接种流感疫苗和 COVID-19 疫苗。
家庭接触者接种减毒活疫苗(包括轮状病毒和水痘疫苗)是安全的。在处理轮状病毒疫苗接种者的脏尿布时,应始终保持彻底的手部卫生,以尽量减少疫苗病毒传播的风险。水痘疫苗接种后,接种者身上出现的任何水痘样水疱都应被遮盖,直至结痂。
其他注意事项
For further information related to specific conditions and vaccination, refer to the Australian Immunisation Handbook.
资源
- MVEC:阿斯普利亚
- MVEC:带状疱疹
- MVEC:实体器官移植受者:移植前免疫建议
- MVEC癌症免疫指南:化疗和造血干细胞移植后的疫苗建议
- MVEC:针对老年人群的免疫建议
- 澳大利亚免疫手册:免疫功能低下者的疫苗接种
- MVEC 电子学习课程:疫苗错误:预防、管理和公开披露 [针对提供者]
作者: Georgina Lewis(默多克儿童研究所 SAEFVIC 临床经理)、Francesca Machingaifa(MVEC 教育护士协调员)和 Rachael McGuire(MVEC 教育护士协调员)
评论: Sally Gordon (MVEC Senior Research Fellow) and Rachael McGuire (MVEC Education Nurse Coordinator)
日期: December 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.
注射部位反应
背景
Injection site reactions (ISRs) are a common localised side effect that can occur following the administration of any injected vaccine. They are an inflammatory response to the injected vaccine. Symptoms of ISRs include swelling, redness (erythema), induration (hardness), pain or itch at or near the injection site.
Less commonly, significant or severe ISRs can present after an injection, extending over a greater area from ‘joint-to-joint’ (e.g. from the shoulder joint to the elbow joint) or ‘crossing joints’ (e.g. swelling that passes over one joint, such as the shoulder or the knee).
High-grade 发烧秒 是 not typically associated with ISRs.
诊断
A GP, immunisation specialist or immunisation provider can diagnose an ISR based on the clinical symptoms and timeline of presentation.
ISRs typically occur within the first 48 hours following vaccination. Symptoms usually last 1 to 2 days and completely resolve within a week. In some rarer instances ISRs can last for a longer period of time (e.g. 5 to 7 days) or have a delayed onset (3 or more days after vaccination).
Photographic evidence and monitoring the circumference of the affected area over time (e.g. draw around the ISR with a pen to see changes in circumference) can assist in documenting the progression of symptoms and support a diagnosis.
Significant ISRs should not be confused with infections such as cellulitis. Symptoms consistent with cellulitis, such as decreased range of movement, lymphangitis (tracking of erythema), lymphadenopathy (swollen lymph nodes) and high-grade fevers, are not consistent with ISRs.
ISRs are not an allergic response.
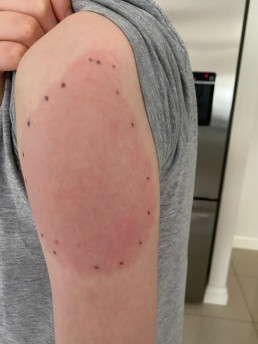
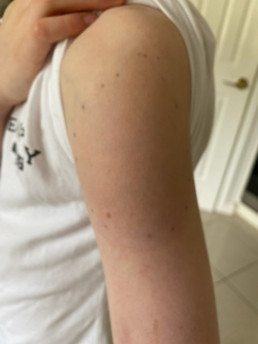
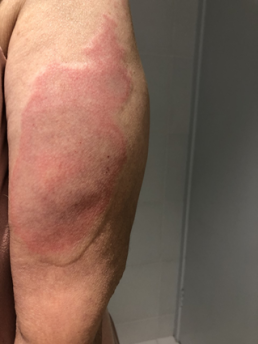
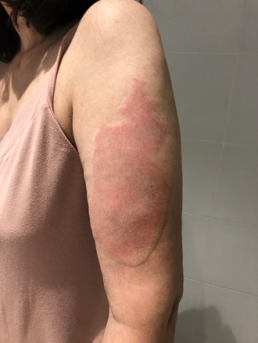
Association and incidence
The true incidence of ISRs is difficult to ascertain as they are often underreported. All injected vaccines have the potential to cause ISR秒. However, factors such as the inclusion of adjuvants in the vaccine ingredients, previous exposure to 一个 antigen (through vaccination) And 这 injection technique used can influence the likelihood of an ISR developing.
Vaccines that contain adjuvants (specific ingredients that are included to evoke stronger immune responses) are often associated with a higher incidence of ISRs. Examples of these vaccines include Shingrix (vaccination against the development of 带状疱疹), 破伤风-containing vaccines (such as Infanrix hexa and Boostrix), 脑膜炎球菌 vaccines (such as Bexsero), 肺炎球菌 vaccines (Pneumovax 23 and Prevenar 13) and adjuvanted 流感 vaccines given to those aged 65 years and over (Fluad Quad and Fluzone High-Dose Quadrivalent).
COVID-19 vaccines and Prevenar 13 are associated with delayed-onset ISRs (occurring more than 3 days after vaccination) when administered to adults 70 years and older.
There is an increased likelihood of ISRs occurring following subsequent doses (primary or boosters) of a particular antigen (e.g. in children aged 18 months and 4 years following diphtheria-tetanus-pertussis boosters, and those receiving subsequent doses of pneumococcal vaccines).
The inadvertent administration of a vaccine into the subcutaneous (SC) tissue, where intramuscular (IM) administration is recommended, has also been associated with an increased incidence of ISR development. Correct injection technique (including route, and appropriate needle size and length) plays an important in mitigating the development of ISRs.
治疗
ISRs will resolve on their own without intervention. They can generally be managed at home with symptomatic relief such as oral analgesia and applying a cold compress to the affected area. Immobilising the affected limb should be avoided; movement will enhance and assist with lymphatic drainage to improve symptoms.
ISRs are not a sign of allergy or local infection. Therefore, antihistamines, steroids or antibiotics are not required.
对未来剂量的影响
While ISRs are a common and expected side effect following most injected vaccines, significant or severe ISRs should be reported to 赛维克 in Victoria.
A previous experience of an ISR is 不是 a contraindication to future doses of the same or any other vaccine. Individuals who have experienced ISRs in the past may experience them again, but they are unlikely to be worse. The benefit of being protected against vaccine-preventable diseases far outweighs the possible risk of developing an ISR. Vaccine recipients who have previously experienced an ISR are encouraged to complete the recommended vaccine schedules.
资源
- MVEC:澳大利亚不良事件报告
- MVEC:SAEFVIC
- MVEC:注射部位结节
- 澳大利亚免疫手册:常见的 AEFI 及其管理方法
- Australian Immunisation Handbook: Table. Comparison of the effects of diseases and the side effects of vaccines on the NIP
- Australian Immunisation Handbook: Vaccination procedures. After vaccination
- NCIRS: Injection site reactions information sheet
作者: Rachael McGuire(默多克儿童研究所 SAEFVIC 研究护士)
审核人: Melissa Humann (SAEFVIC Research Nurse, Murdoch Children’s Research Institute), Rachael McGuire (MVEC Education Nurse Coordinator) and Katie Butler (MVEC Education Nurse Coordinator)
日期: December 2023
本章节内的材料将随着新信息和新疫苗的出现而进行更新。墨尔本疫苗教育中心(MVEC)职员定期审阅材料的准确性。
You should not consider the information on this site to be specific, professional medical advice for your personal health or for your family’s personal health. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult a healthcare professional.